Antibiotic-resistant bacterial infections are becoming a major societal challenge. To solve this problem, researchers are working on new drugs that kill bacteria without developing resistance, and on new materials that prevent the formation of bacterial biofilms. The latter are the source of resistant variants but are notoriously difficult to remove. Recently, researchers from the Institute of Physical Chemistry, Polish Academy of Sciences have discovered that making the surface undulated can help limit the spread of resistant bacteria.

Credit: Photo copyright: Karol Karnowski
Antibiotic-resistant bacterial infections are becoming a major societal challenge. To solve this problem, researchers are working on new drugs that kill bacteria without developing resistance, and on new materials that prevent the formation of bacterial biofilms. The latter are the source of resistant variants but are notoriously difficult to remove. Recently, researchers from the Institute of Physical Chemistry, Polish Academy of Sciences have discovered that making the surface undulated can help limit the spread of resistant bacteria.
The global overuse of antibiotics has significantly sped up the development of resistant bacteria, which are quickly spreading world-wide and replacing antibiotic-sensitive bacteria. Many commonly-occurring strains of pathogenic bacteria are now resistant to multiple antibiotics, making it imperative to find new methods of combatting bacterial infections. In the search for a way to limit the growth of undesired resistant bacteria, researchers from the Dioscuri Centre for Physics and Chemistry of Bacteria, IPC PAS, look at the behaviour of antibiotic-sensitive and antibiotic-resistant E. coli bacteria on different surfaces. Researchers hope that a better understanding of how such bacterial populations grow will help develop novel antibacterial coatings that prevent the emergence of resistance.
In nature as well as in the human body, bacteria often form closely-knit, surface-attached communities called biofilms. Bacteria in biofilms are partially protected from antibiotics, which cannot penetrate the biofilm due to its dense structure. As in the aphorism “what does not kill you makes you stronger”, this encourages the evolution of antibiotic-resistant bacteria that spontaneously emerge in the biofilm. Following antibiotics exposure such as during an infection, a few resistant bacteria that initially emerged begin to multiply because the antibiotic is unable to kill them. If this process occurs in a biofilm growing on a flat surface, for example that of a urinary catheter, nothing prevents resistant bacteria to spread and eventually take over the sensitive bacteria.
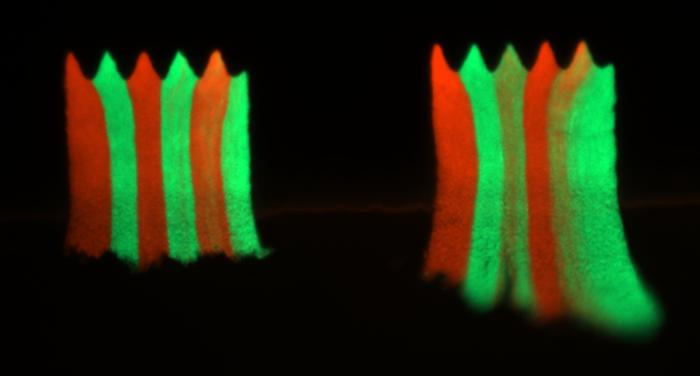
Researchers from IPC PAS have now demonstrated that this process can be arrested by making the surface corrugated. In a recent article published in the prestigious journal PNAS https://www.eurekalert.org/news-releases/1049682, they show that even tiny surface undulations, much smaller than the thickness of the biofilm, are sufficient to limit the spread of resistant mutants to tiny “sectors” within the biofilm.
The team led by Dr Bartłomiej Wacław grew bacterial biofilms in a microfluidic device composed of microscopic chambers with a corrugated bottom surface in the shape of a sine function. Each chamber was occupied by a biofilm made of two different bacteria, sensitive and resistant, which were initially mixed together. Following the exposure to an antibiotic, resistant bacteria began to grow faster than sensitive bacteria. However, in contrast to what happened in flat-bottom chambers, resistant bacteria did not take over the sensitive ones in corrugated chambers. Instead, resistant bacteria remained localized to small sectors located within the pockets of the corrugated pattern.
“Our results suggest that bacterial population dynamics in the biofilm can be controlled by manipulating the surface geometry. We have demonstrated this specifically for an antibiotic-resistant mutant, which has potentially interesting implications for medicine. Patterned surfaces have already been investigated for their anti-biofilm forming potential. Our research provides another rationale for the use of such surfaces – they can also prevent the spread of resistant bacteria.” – claims Dr Wacław.
Dr Waclaw stresses, however, that it is too early to say whether the basic research carried out in his group will translate into practical applications. He points out, however, that “while this approach will certainly not eliminate the problem of antibiotic resistance, it may help reduce the risk of therapy failure in hospitalized patients. Such patients are often catheterized, which carries a significant risk of an infection with a hospital-dwelling antibiotic-resistant strain. Slowing the spread of such bacteria may mean the difference between life and death for some patients”.
Dr Waclaw also says that their result is more general and applies to any situation in which an undesired the bacterial strains grows faster than other strains. “A similar effect could be exploited to stabilize microbial communities used in industrial processes such as waste water treatment, biofuel production, and many others”.
This work was supported by the Bekker Programme of the Polish National Agency for Academic Exchange (NAWA) grant no. PPN/BEK/2020/1/00333/U/00001, the START scholarship of the Foundation for Polish Science grant no. 069.2021, NAWA Polish Returns grant no. PPN/PPO/2019/1/00030/U/0001, and Dioscuri, a program initiated by the Max Planck Society, jointly managed with the National Science Centre in Poland, and mutually funded by the Polish Ministry of Science and Higher Education and German Federal Ministry of Education and Research, grant no. UMO-2019/02/H/NZ6/00003