A new study reviews how cancer-associated fibroblasts (CAFs) influence lymph node metastasis. Researchers identified various CAF subsets that interact with the tumor microenvironment, promoting cancer spread. By targeting these specific subsets, new therapeutic strategies can be developed to inhibit metastasis, potentially improving patient outcomes. This breakthrough offers a deeper understanding of CAF heterogeneity and its role in cancer progression, paving the way for more effective cancer treatments.
Lymph node metastasis is a crucial factor in cancer progression, significantly affecting patient prognosis and treatment outcomes. The tumor microenvironment, particularly cancer-associated fibroblasts (CAFs), plays a vital role in this process. CAFs influence the metastatic landscape through interactions with other cells and secretion of signaling molecules. Based on these challenges, it is essential to conduct in-depth research to uncover the molecular mechanisms involving CAFs and develop targeted therapies that can inhibit lymphatic metastasis and improve cancer treatment strategies.
Researchers from Sun Yat-sen Memorial Hospital, publishing a study (DOI: 10.20892/j.issn.2095-3941.2024.0138) in Cancer Biology & Medicine on May 22, 2024, have reviewed the heterogeneity of CAFs and their roles in promoting lymphatic metastasis. This study provides a comprehensive analysis of how different CAF subsets interact with the tumor microenvironment, offering new strategies for targeted cancer therapies.
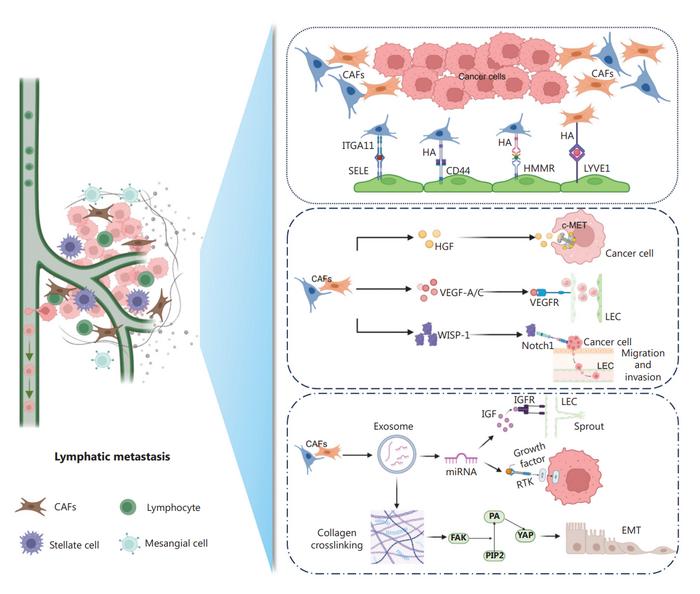
This study highlights the crucial role of CAFs in shaping the lymphatic metastatic landscape. Researchers reviewed various CAF subsets that interact with other stromal cells, secrete cytokines, and produce exosomes, all of which contribute to lymphatic invasion and metastasis. One significant finding is the role of PDGFRα+ITGA11+ CAFs in bladder cancer, which directly interact with lymphatic endothelial cells, enhancing lymphovascular invasion and metastasis. The study also explores the therapeutic potential of targeting specific CAF subsets, showing promising results in preclinical models. By inhibiting the interactions between CAFs and other cells, particularly through targeted antibodies, researchers demonstrated a reduction in lymphatic metastasis. These findings offer valuable insights into the complex mechanisms of CAF-mediated metastasis and suggest new avenues for developing targeted cancer therapies.
Dr. Changhao Chen, a lead researcher, stated, “Our findings underscore the complexity and significance of CAF heterogeneity in cancer progression. By targeting specific CAF subsets, we can develop more effective therapeutic strategies to inhibit lymphatic metastasis and improve patient outcomes.”
The insights from this study pave the way for developing novel cancer therapies that specifically target CAFs. By understanding the diverse roles of CAFs in tumor metastasis, researchers can design targeted treatments that potentially reduce the spread of cancer and enhance the efficacy of existing therapies. This approach could lead to improved prognoses and quality of life for cancer patients.

Credit: Cancer Biology & Medicine
A new study reviews how cancer-associated fibroblasts (CAFs) influence lymph node metastasis. Researchers identified various CAF subsets that interact with the tumor microenvironment, promoting cancer spread. By targeting these specific subsets, new therapeutic strategies can be developed to inhibit metastasis, potentially improving patient outcomes. This breakthrough offers a deeper understanding of CAF heterogeneity and its role in cancer progression, paving the way for more effective cancer treatments.
Lymph node metastasis is a crucial factor in cancer progression, significantly affecting patient prognosis and treatment outcomes. The tumor microenvironment, particularly cancer-associated fibroblasts (CAFs), plays a vital role in this process. CAFs influence the metastatic landscape through interactions with other cells and secretion of signaling molecules. Based on these challenges, it is essential to conduct in-depth research to uncover the molecular mechanisms involving CAFs and develop targeted therapies that can inhibit lymphatic metastasis and improve cancer treatment strategies.
Researchers from Sun Yat-sen Memorial Hospital, publishing a study (DOI: 10.20892/j.issn.2095-3941.2024.0138) in Cancer Biology & Medicine on May 22, 2024, have reviewed the heterogeneity of CAFs and their roles in promoting lymphatic metastasis. This study provides a comprehensive analysis of how different CAF subsets interact with the tumor microenvironment, offering new strategies for targeted cancer therapies.
This study highlights the crucial role of CAFs in shaping the lymphatic metastatic landscape. Researchers reviewed various CAF subsets that interact with other stromal cells, secrete cytokines, and produce exosomes, all of which contribute to lymphatic invasion and metastasis. One significant finding is the role of PDGFRα+ITGA11+ CAFs in bladder cancer, which directly interact with lymphatic endothelial cells, enhancing lymphovascular invasion and metastasis. The study also explores the therapeutic potential of targeting specific CAF subsets, showing promising results in preclinical models. By inhibiting the interactions between CAFs and other cells, particularly through targeted antibodies, researchers demonstrated a reduction in lymphatic metastasis. These findings offer valuable insights into the complex mechanisms of CAF-mediated metastasis and suggest new avenues for developing targeted cancer therapies.
Dr. Changhao Chen, a lead researcher, stated, “Our findings underscore the complexity and significance of CAF heterogeneity in cancer progression. By targeting specific CAF subsets, we can develop more effective therapeutic strategies to inhibit lymphatic metastasis and improve patient outcomes.”
The insights from this study pave the way for developing novel cancer therapies that specifically target CAFs. By understanding the diverse roles of CAFs in tumor metastasis, researchers can design targeted treatments that potentially reduce the spread of cancer and enhance the efficacy of existing therapies. This approach could lead to improved prognoses and quality of life for cancer patients.
###
References
DOI
10.20892/j.issn.2095-3941.2024.0138
Original Source URL
Funding information
This work was funded by the National Key Research and Development Program of China (Grant No. 2022YFA1305500), the National Natural Science Foundation of China (Grant Nos. 32322023, 82173272, 81825016, and 82173230), the Key Areas Research and Development Program of Guangdong (Grant Nos. 2022B1515120086 and 2022A1515140175), and the Science and Technology Program of Guangzhou, China (Grant No. 2023A04J2206).
About Cancer Biology & Medicine
Cancer Biology & Medicine (CBM) is a peer-reviewed open-access journal sponsored by China Anti-cancer Association (CACA) and Tianjin Medical University Cancer Institute & Hospital. The journal monthly provides innovative and significant information on biological basis of cancer, cancer microenvironment, translational cancer research, and all aspects of clinical cancer research. The journal also publishes significant perspectives on indigenous cancer types in China. The journal is indexed in SCOPUS, MEDLINE and SCI (IF 5.6, 5 year IF 5.9), with all full texts freely visible to clinicians and researchers all over the world (http://www.ncbi.nlm.nih.gov/pmc/journals/2000/).
Journal
Cancer Biology & Medicine
Subject of Research
Not applicable
Article Title
Roles of cancer-associated fibroblast functional heterogeneity in shaping the lymphatic metastatic landscape: new insights and therapeutic strategies
Article Publication Date
22-May-2024
COI Statement
The authors declare that they have no competing interests.