This study investigates the cononsolvency mechanism of poly(N-isopropylacrylamide) (PNIPAM), which is soluble in pure methanol (MeOH) and water but insoluble in aqueous MeOH solutions. Combining oxygen K-edge X-ray absorption spectroscopy (XAS) with theoretical calculations executed in molecular dynamics (MD) simulations and inner-shell calculations, it was found that hydrophobic interactions between PNIPAM and MeOH clusters play a key role in PNIPAM aggregation and cononsolvency emergence.

Credit: Masanari Nagasaka
This study investigates the cononsolvency mechanism of poly(N-isopropylacrylamide) (PNIPAM), which is soluble in pure methanol (MeOH) and water but insoluble in aqueous MeOH solutions. Combining oxygen K-edge X-ray absorption spectroscopy (XAS) with theoretical calculations executed in molecular dynamics (MD) simulations and inner-shell calculations, it was found that hydrophobic interactions between PNIPAM and MeOH clusters play a key role in PNIPAM aggregation and cononsolvency emergence.
PNIPAM is a stimuli-responsive polymer showing sensitivity to various chemical environments such as temperature and pH. PNIPAM dissolves in pure MeOH and H2O at room temperature but is insoluble in mixtures of MeOH and H2O, a phenomenon known as cononsolvency. Understanding the mechanism of cononsolvency is important for comprehending the phase transition dynamics not only of polymers but also of biomolecules, which undergo dynamics such as protein folding, DNA packing, and interchain complexation. In this study, we investigated the cononsolvency mechanism of PNIPAM in aqueous MeOH solutions from the oxygen K-edge XAS of PNIPAM along with theoretical calculations implemented in MD simulations and inner-shell calculations.
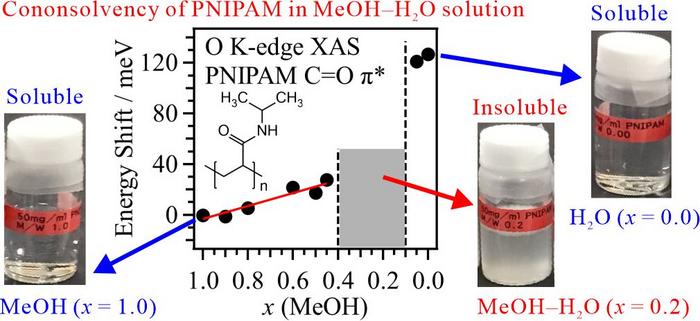
The oxygen K-edge XAS spectra of PNIPAM were measured in a transmission-type liquid cell at the soft X-ray beamline BL-7A of the Photon Factory (KEK-PF). XAS enables the element-selective analysis of light elements such as carbon, nitrogen, and oxygen. However, XAS measurements in transmission mode are difficult because soft X-rays are strongly absorbed by air and liquids. Our developed liquid cell allows XAS measurements of liquid samples in transmission mode under precise thickness control. The C=O π* peaks in the PNIPAM spectrum were observed after separating the contributions of the MeOH and H2O solvents. Figure 1 plots the energy shift of the C=O π* peaks in PNIPAM as a function of MeOH molar fraction at 25°C. n the MeOH-rich region, the energy shifts of the C=O π* peaks are higher in the mixed solvent than in pure MeOH. This energy shift is assigned to simple substitution of the hydrogen bond (HB) structure of the PNIPAM C=O group from MeOH to H2O. In contrast, the energy shift of the C=O π* peak of PNIPAM is much higher in pure H2O than in pure MeOH. Although the dissolution behaviors of PNIPAM in H2O and MeOH are identical on the macroscopic scale, the molecular interactions of PNIPAM with H2O and MeOH are very different on the microscopic scale. For this reason, cononsolvency of PNIPAM emerges in aqueous MeOH solutions.
To reveal the origin of the energy shift of the C=O π* peak in the PNIPAM XAS spectrum, we investigated the structures of PNIPAM chains in aqueous MeOH solutions through MD simulations. The model structures of the HBs between PNIPAM and the MeOH and H2O solvents were determined from the radial distribution functions in the MD simulations and were utilized in the inner-shell calculations. Comparing the inner-shell spectra with the experimentally obtained XAS spectra of PNIPAM, we found that PNIPAM forms rounded structures in pure H2O but chain structures in pure MeOH. This finding explains the much higher energy shift of the C=O π* peak of PNIPAM in pure H2O than in pure MeOH. In the rounded form in pure H2O, the isopropyl group in PNIPAM undergoes hydrophobic hydration. The cononsolvency in aqueous MeOH solutions emerges from hydrophobic interactions between PNIPAM and MeOH clusters, which disrupt the hydrophobic hydration of PNIPAM and induce PNIPAM aggregation. The present study confirmed the applicability of element-selective XAS analysis to phase transition dynamics of both polymers and biomolecules, where the latter include protein folding, DNA packing, and interchain complexation.
Information of the paper:
Authors: Masanari Nagasaka, Fumitoshi Kumaki, Yifeng Yao, Jun-ichi Adachi and Kenji Mochizuki
Journal Name: Physical Chemistry Chemical Physics
Journal Title: “Mechanism of poly(N-isopropylacrylamide) cononsolvency in aqueous methanol solutions explored via oxygen K-edge X-ray absorption spectroscopy”
DOI:
Financial Supports:
JSPS, KAKENHI, Fostering Joint International Research (B): JP19H02680
National Natural Science Foundation of China: No. 22273083, 22250610195
KEK, Photon Factory Program Advisory Committee: No. 2021G047
NINS, Okazaki Research Facilitie, Research Center for Computational Science: 22-IMS-C187
Contact Person:
Masanari Nagasaka
Institute for Molecular Science, NINS
The Graduate University for Advanced Studies, SOKENDAI
TEL/FAX: +81-564-55-7394 / +81-564-55-7493
E-mail: nagasaka_at_ims.ac.jp(Please replace the “_at_” with @)
Journal
Physical Chemistry Chemical Physics
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Mechanism of poly(N-isopropylacrylamide) cononsolvency in aqueous methanol solutions explored via oxygen K-edge X-ray absorption spectroscopy
Article Publication Date
17-Apr-2024