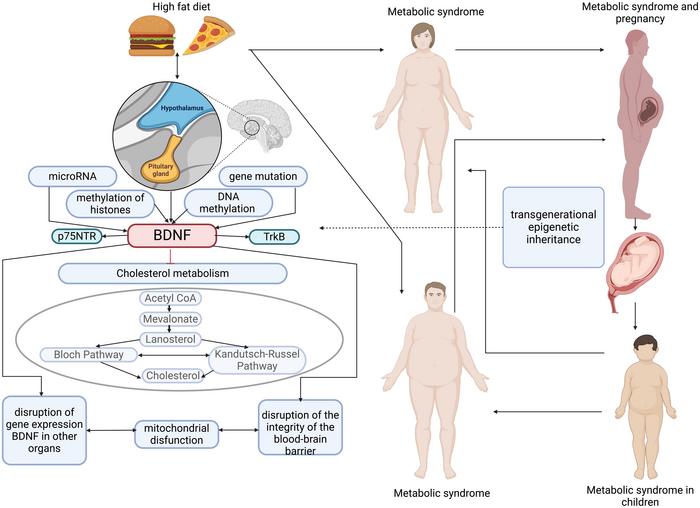
Metabolic syndrome (MetS) is a multifaceted disorder that impacts approximately 20–25% of the global population. This syndrome encompasses a range of conditions, including obesity, type 2 diabetes mellitus, hyperinsulinemia, insulin resistance (IR), hypercholesterolemia, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and systemic metabolic inflammation. These conditions collectively lead to severe diseases and increased premature mortality. The hypothalamus, a critical brain structure regulating eating behavior, plays a pivotal role in the development of MetS. The connection between psychoneurotic disorders and MetS underscores the significant role of the brain in the syndrome’s progression. Additionally, MetS is associated with cancer development, likely mediated by hypothalamic dysfunction.

Credit: Olga Khaziakhmatova, Natalia Todosenko
Metabolic syndrome (MetS) is a multifaceted disorder that impacts approximately 20–25% of the global population. This syndrome encompasses a range of conditions, including obesity, type 2 diabetes mellitus, hyperinsulinemia, insulin resistance (IR), hypercholesterolemia, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and systemic metabolic inflammation. These conditions collectively lead to severe diseases and increased premature mortality. The hypothalamus, a critical brain structure regulating eating behavior, plays a pivotal role in the development of MetS. The connection between psychoneurotic disorders and MetS underscores the significant role of the brain in the syndrome’s progression. Additionally, MetS is associated with cancer development, likely mediated by hypothalamic dysfunction.
The hypothalamus is integral to energy homeostasis and metabolism regulation. Inflammation and alterations in hypothalamic function can precipitate MetS. In animal studies, a high-fat diet (HFD) in mothers results in hypothalamic inflammation and gliosis in offspring, contributing to metabolic dysregulation. The blood-brain barrier (BBB) integrity is compromised, allowing inflammatory mediators and fatty acids to affect fetal hypothalamus development. These alterations lead to changes in neuronal communication, increased expression of inflammatory markers, and disruptions in insulin and leptin signaling pathways, promoting obesity and insulin resistance in offspring.
Maternal and paternal obesity can epigenetically reprogram offspring, affecting their metabolic health. Maternal HFD exposure leads to significant changes in the hypothalamus of the offspring, including increased expression of genes associated with inflammation and altered neuronal signaling. These changes are sex-specific, with female offspring exhibiting greater susceptibility to metabolic disorders. Paternal obesity similarly influences offspring through epigenetic modifications, such as hypomethylation of growth-regulating genes, contributing to metabolic dysfunctions.
The maternal hypercaloric diet affects lipid metabolism and the endogenous cannabinoid system in the hypothalamus of adult offspring, leading to sex-specific metabolic responses. This diet induces an increase in the expression of lipid metabolism-related genes and cannabinoid receptors in the hypothalamus. Such changes disrupt normal metabolic processes, contributing to the development of obesity and other metabolic disorders in offspring.
Developmental programming plays a crucial role in determining the susceptibility to metabolic disorders. During critical periods of development, exposure to adverse environmental factors, such as a hypercaloric diet, can permanently alter hypothalamic structure and function. These changes predispose individuals to metabolic disorders later in life. Epigenetic mechanisms, including DNA methylation and histone modifications, mediate these long-lasting effects. For example, miRNAs are crucial in regulating hypothalamic development and function, with their expression being influenced by maternal diet.
The hypothalamus contains several key signaling pathways that regulate energy balance and metabolism. The Notch signaling pathway, for instance, is involved in hypothalamic neurogenesis and is disrupted in the offspring of obese mothers, leading to altered neuronal development and metabolic dysfunction. Similarly, the POMC neurons in the hypothalamus, which play a critical role in regulating appetite and energy expenditure, are affected by maternal obesity and hyperglycemia, resulting in increased food intake and weight gain in offspring.
Metabolic syndrome’s complexity is underscored by the intricate interplay between genetic, epigenetic, and environmental factors. The hypothalamus’s central role in regulating metabolism and the significant impact of parental obesity on offspring’s metabolic health highlight the importance of addressing these factors to mitigate the rising prevalence of MetS. Understanding the epigenetic and developmental mechanisms involved provides insights into potential therapeutic targets for preventing and managing metabolic disorders.
Full text
The study was recently published in the Gene Expression.
Gene Expression (GE) is an open-access journal. It was launched in 1991 by Chicago Medical School Press, and transferred to Cognizant Communication Corporation in 1994. From August 2022, GE is published by Xia & He Publishing Inc.
GE publishes peer-reviewed and high-quality original articles, reviews, editorials, commentaries, and opinions on its primary research topics including cell biology, molecular biology, genes, and genetics, especially on the cellular and molecular mechanisms of human diseases.
GE has been indexed in Medline (1991-2021), Scopus, Biological Abstracts, Biosis Previews, ProQuest, etc.
Follow us on X: @xiahepublishing
Follow us on LinkedIn: Xia & He Publishing Inc.
Journal
Gene Expression
Article Title
(Epi)genetic Aspects of Metabolic Syndrome Pathogenesis in Relation to Brain-derived Neurotrophic Factor Expression: A Review
Article Publication Date
8-May-2024