Ischemic stroke represents a critical health crisis globally, inflicting immense burdens of mortality and long-term disability across diverse populations. Following such an event, the brain’s extracellular space (ECS) becomes a site of significant biochemical turmoil, wherein the accumulation of pathological biomolecules occurs. These toxic substances worsen neurological damage and impair cognitive functions, signaling an urgent need for innovative therapeutic strategies. Recent studies have highlighted the potential of photobiomodulation (PBM) as a promising intervention, which may alter the accumulation dynamics of these biomolecules and thus mitigate the adverse outcomes associated with ischemic stroke.
The therapeutic approach utilizing PBM is based on its ability to invigorate cerebral functions by enhancing the transportation of critical molecules within the ECS. Researchers, including study lead Hongbin Han from Peking University Third Hospital, emphasize the therapeutic promise of PBM, particularly in its ability to ameliorate cognitive decline in Alzheimer’s disease models by expediting molecular flows within the ECS. This discovery suggests that PBM may be beneficial in countering the detrimental accumulation of harmful substances in the ECS following an ischemic stroke, a hypothesis that requires rigorous examination.
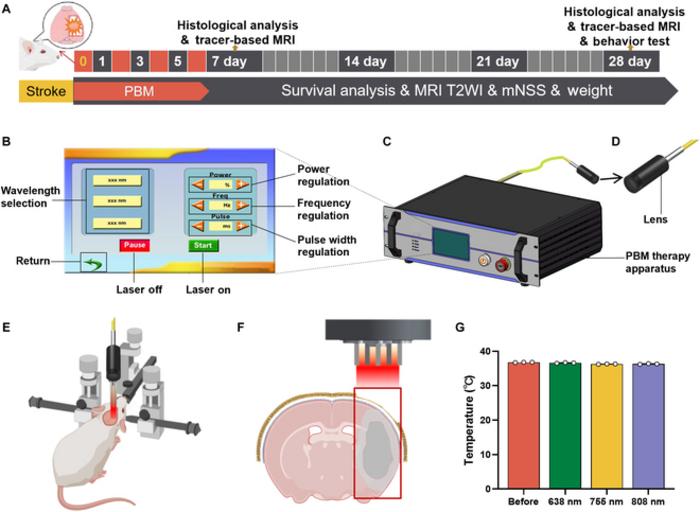
Recent advancements reveal that PBM, particularly at a wavelength of 755 nm, can improve the drainage of interstitial fluid (ISF) while fostering molecular transport in the ECS following ischemic insults. By conducting meticulous experiments involving transient middle cerebral artery occlusion (tMCAO) in rat models, researchers have delineated the effects of targeted 755 nm light exposure on post-stroke recovery. The use of precise imaging techniques has allowed researchers to verify the efficiency of PBM in enhancing ISF drainage, making the clear identification of pathways involved in the neuroprotective effects essential for understanding this modality’s therapeutic potential.
The research team’s findings conclusively show that PBM therapy accelerates the transport of neuroprotective and anti-inflammatory mediators within the ECS. By efficiently promoting the clearance of pro-inflammatory cytokines and reducing the accumulation of pathological proteins, PBM leads to a decrease in infarct volume, subsequently translating into enhanced neurological and cognitive functions. Notably, the accelerated drainage of ISF observed in subjects undergoing PBM was linked to a modification in aquaporin 4 (AQP4) expression—mirroring a restoration of the polarization necessary to facilitate fluid exchange and clearance in brain tissue.
Furthermore, during the PBM sessions, the experimental setup involved the placement of a specialized apparatus designed for optimal light delivery precisely over the damaged brain area. This innovative therapy design comprised a touchscreen control unit alongside a lens to focus light, enhancing treatment localization. The meticulous approach used in this study attests to the precision with which PBM can be deployed, ensuring that therapeutic light penetrates the brain tissue effectively while minimizing any potential adverse effects.
Significantly, the findings from this research support the notion that PBM exerts its neuroprotective effects not solely through direct neuronal interactions but also via modulations within the ECS that can impact larger neurobiological mechanisms. This perspective opens avenues for PBM to serve as a cornerstone in developing comprehensive treatment protocols for ischemic strokes, challenging traditional paradigms and embracing innovative methodologies. The implications of these results extend beyond immediate therapeutic effects—hinting at a future where light-based therapies redefine stroke recovery processes significantly.
Moreover, the multifaceted benefits observed with PBM suggest that the mechanism of action may involve reducing necrotic cell death and promoting survival pathways. Such findings align with an evolving understanding of the brain’s regenerative responses to injury, thus fostering renewed hope for patients affected by ischemic stroke. As researchers continue to unravel the complexities of this treatment approach, the therapeutic landscape for stroke intervention may witness a transformative shift.
The meticulous nature of this study underscores not just the efficacy of PBM but also the promising technological endeavors surrounding its application. By developing specific parameters for PBM treatment, the research paves the way for targeted trials that could establish standardized practices and broaden the scope of PBM applications across various brain injury contexts beyond stroke. Future research could expand on these findings, optimizing treatment modalities while investigating their synergistic potential alongside existing clinical practices.
In conclusion, this research represents a significant advance in understanding how PBM therapy can effectively alleviate post-stroke cognitive impairment. The study’s insights present a nuanced comprehension of the interaction between PBM and the ECS, identifying actionable pathways towards developing effective interventions for stroke patients. Progressing from bench to bedside, researchers are poised to harness these findings, offering a novel, non-invasive, and effective strategy for addressing one of the most pressing health crises of our time.
With continuous funding and ongoing collaborative efforts, the journey towards integrating PBM into clinical practice appears promising. As more is understood about the mechanisms at play and the best practices for implementing PBM effectively, the vision of a future where ischemic strokes result in minimal long-term cognitive impairment becomes progressively attainable.
Harnessing the principles behind this innovation and understanding the underpinnings of its effects could yield transformative insights—not just for stroke recovery but potentially for various neurodegenerative conditions, thereby broadening the ramifications of light-based therapies across the medical landscape.
Subject of Research: Photobiomodulation Therapy for Ischemic Stroke
Article Title: Accelerated Molecular Transportation in the Brain Extracellular Space with 755-nm Light Attenuates Post-Stroke Cognitive Impairment in Rats
News Publication Date: May 6, 2025
Web References: DOI: 10.34133/cbsystems.0262
References: National Natural Science Foundation of China grants; Science and Technology Program of Guangzhou; China Postdoctoral Science Foundation; various authors.
Image Credits: Hongbin Han, Department of Radiology, Peking University Third Hospital
Keywords
PBM, Ischemic Stroke, Cognitive Impairment, Neuroscience, Aquaporin 4, Phototherapy, Brain Extracellular Space, Molecular Transportation, Interstitial Fluid Drainage.