The fight against respiratory viruses, particularly respiratory syncytial virus (RSV), has been continuously evolving as researchers delve into the molecular intricacies of viral infections. One of the most critical players in the cellular response to viral infections is a family of enzymes known as DNA methyltransferases. Among these, DNA methyltransferase 3 alpha (DNMT3A) has garnered significant attention in recent years due to its pivotal role in epigenetic regulation and gene expression. The study conducted by Becker et al. marks a significant advance in understanding the expression dynamics of DNMT3A in the context of RSV strain A infection, elucidating a pathway that could lead to novel therapeutic approaches.
Respiratory syncytial virus is a leading cause of respiratory infections in infants and young children, with the potential to cause severe respiratory distress and even hospitalization. The virus has a complex life cycle that hijacks host cellular machinery to replicate and propagate, often leading to significant immune responses. These responses, while crucial for controlling the infection, can also drive pathology. By examining the dynamic expression of DNMT3A during RSV infection, the researchers aim to uncover how this enzyme might influence viral replication and the host immune response.
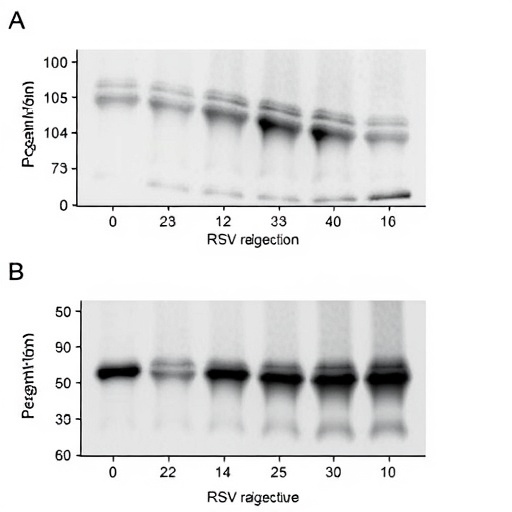
The methodology employed by Becker et al. was thorough and multifaceted. By utilizing in vitro models of viral infection, the researchers were able to isolate and assess the expression levels of DNMT3A at various points during the replication cycle. Quantitative PCR and Western blotting techniques provided robust data regarding mRNA and protein expression, respectively, allowing for a clear picture of how DNMT3A levels fluctuate upon viral exposure. Additionally, the use of RNA interference techniques provided insights into the functional role of DNMT3A in mediating the host response to RSV.
One of the key findings of the study revealed that DNMT3A expression is markedly upregulated in response to RSV strain A infection. This increase suggests that DNMT3A may be part of a host defense mechanism geared towards regulating genes involved in the antiviral response. Concurrently, the expression of various pro-inflammatory cytokines was also assessed, establishing a link between DNMT3A activity and the host’s immune signaling pathways. Such findings highlight the dual role of DNMT3A not only in gene regulation but also in shaping the immune landscape during viral invasions.
Moreover, the study delved deeper into the specific pathways by which DNMT3A influences gene expression. Through the analysis of methylation patterns on viral and host genomic DNA, the researchers outlined how changes in DNMT3A activity correspond to alterations in the methylation landscape. These modifications can directly influence gene expression levels, thereby affecting the efficiency of viral replication and the host’s ability to mount an effective immune response.
Notably, the researchers also explored the implications of DNMT3A knockdown on viral replication rates. By silencing DNMT3A expression, the team observed a marked decrease in viral titers, underscoring the enzyme’s essential role in supporting RSV’s life cycle. This finding not only adds a layer of understanding to the host-virus interaction but also points to the potential of targeting DNMT3A as a therapeutic strategy. If DNMT3A is indeed a facilitator of RSV replication, inhibiting its activity could enhance treatment outcomes in infected patients.
Another important dimension of this research is its relevance to developing antiviral therapies. With the rise of drug-resistant strains and the limited effectiveness of current antiviral agents against RSV, the identification of new targets for pharmacological intervention is imperative. By providing a detailed analysis of DNMT3A’s role in RSV infection, Becker et al. open the door for the next generation of antiviral drugs that could specifically modulate DNMT3A activity or its epigenetic regulation.
The preliminary data presented by Becker et al. shed light on the broader implications of viral infections on epigenetic regulation. As more studies converge on the intersection of epigenetics and virology, it becomes increasingly clear that viruses employ sophisticated mechanisms to manipulate host cellular machinery. This manipulation often goes beyond immediate viral needs, influencing long-term cellular states and responses to subsequent infections. Hence, understanding DNMT3A’s role becomes crucial in constructing a holistic view of virus-host interactions.
Collectively, the findings from Becker et al. reinforce the importance of epigenetic factors in viral pathogenesis. The study highlights how viruses can exploit host epigenetic machinery to promote their replication and evade immune surveillance. This relationship opens new avenues for research focused on identifying additional epigenetic markers influenced by viral infections and their potential as targets for immunotherapeutic strategies.
In conclusion, the rigorous investigation into DNA methyltransferase 3 alpha (DNMT3A) expression during respiratory syncytial virus strain A infection has yielded promising insights that could transform our understanding of host-virus dynamics. The potential to manipulate DNMT3A activity for therapeutic benefit represents an exciting frontier in respiratory virus research and underscores the broader significance of epigenetic modulation in infectious diseases. As the research community continues to probe the intricate mechanisms of viral infections, studies like those conducted by Becker and colleagues will be pivotal in paving the way for innovative treatment solutions.
Subject of Research: The expression of DNA methyltransferase 3 alpha during respiratory syncytial virus strain A infection.
Article Title: Analysis of DNA methyltransferase 3 alpha expression during respiratory syncytial virus strain A infection.
Article References:
Becker, A.L., Borges, S.G., Pinheiro, L.G.R. et al. Analysis of DNA methyltransferase 3 alpha expression during respiratory syncytial virus strain A infection.
Sci Rep (2025). https://doi.org/10.1038/s41598-025-34030-2
Image Credits: AI Generated
DOI: 10.1038/s41598-025-34030-2
Keywords: respiratory syncytial virus, DNA methyltransferase, epigenetics, host-virus interaction, antiviral therapy.