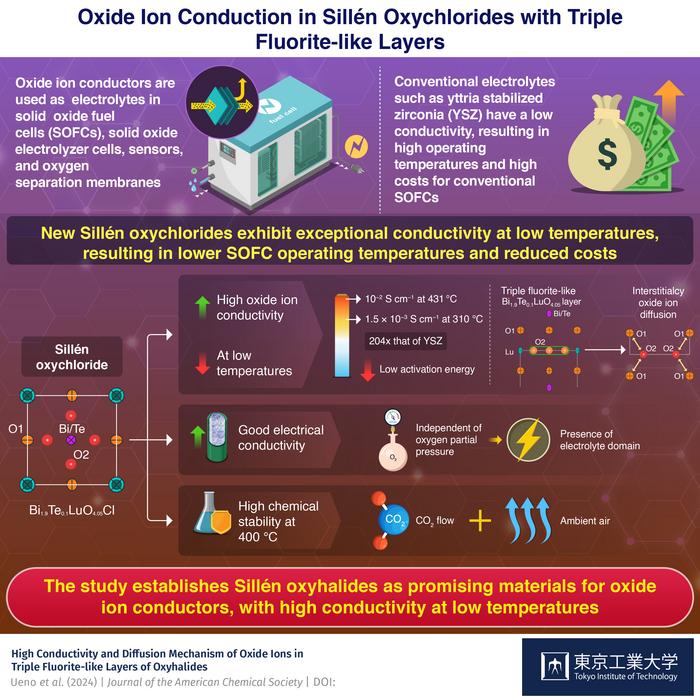
Oxide ion conductors used in solid-state fuel cells often fail to reach full potential when operating at temperatures below 500 oC, but researchers from Tokyo Tech have recently found a solution to this problem. They demonstrated high conductivity and stability in bismuth-containing Sillén oxyhalides with triple fluorite-like layers (e.g. 10 mS/cm at 431 oC; 204 times higher conductivity than that of conventional conductors at 310 oC).

Credit: Tokyo Institute of Technology
Oxide ion conductors used in solid-state fuel cells often fail to reach full potential when operating at temperatures below 500 oC, but researchers from Tokyo Tech have recently found a solution to this problem. They demonstrated high conductivity and stability in bismuth-containing Sillén oxyhalides with triple fluorite-like layers (e.g. 10 mS/cm at 431 oC; 204 times higher conductivity than that of conventional conductors at 310 oC).
Solid oxide fuel cells (SOFCs) are known for their high efficiency and improved safety compared to other types of fuel cells. One of the key factors in SOFC performance is the solid electrolyte: the oxide ion conductor. Its excellent electrochemical properties make it an ideal electrolyte not only for SOFC applications but also for solid oxide electrolyzer cells (SOECs), sensors and oxygen separation membranes.
Despite the significant advantages of oxide ion conductors, commonly used oxide ion conductors such as yttria-stabilized zirconia (YSZ) require extremely high operating temperatures of 1000−700 oC. Over long periods, such high temperatures can be detrimental to SOFC performance. To prevent degradation, expensive heat-resistant alloys are used, which automatically increases the production cost of SOFCs. In addition, there is a lack of stable oxide ion conductors that exhibit a conductivity of 10−2 S cm−1 below 500 oC.
To bridge the existing gap in the stable oxide ion electrolytes with lower operating temperatures, a team of researchers from Tokyo Institute of Technology (Tokyo Tech), led by Professor Masatomo Yashima, recently turned their attention to Sillén oxyhalides. In the latest Journal of the American Chemical Society study, they synthesized a series of bismuth (Bi)-containing Sillén oxyhalides and investigated their electrical and structural properties. “We chose materials containing Bi species because they are known to exhibit high oxide ion conductivity. Additionally, the parent materials Sillén oxyhalides possess triple fluorite-like layers with interstitial oxygen sites, which can lead to interstitialcy oxide ion diffusion,” says Yashima when asked about their choice of materials.
In this study, the researchers synthesized Sillén oxychlorides with the molecular formula Bi2−xTexLuO4+x/2Cl (x = 0, 0.1, and 0.2). The team’s experiments on Bi1.9Te0.1LuO4.05Cl showed high bulk conductivity of 10 mS/cm (= 0.01 Ω−1 cm−1), which is a standard for practical use in fuel cells, at a much lower temperature of 431 oC than 644 oC of the conventional material YSZ (Yttria-Stabilized Zirconia). Bi1.9Te0.1LuO4.05Cl also exhibits high bulk conductivity of 1.5 × 10−3 S cm−1 at a low temperature of 310 °C, which is more than 200 times higher than that of YSZ. This behavior was attributed to the low activation energy. Further analysis using neutron diffraction experiments, DFT calculations, and molecular dynamics simulations revealed that the low activation energy and high conductivity emerge due to the presence of interstitialcy oxide ion diffusion in the triple fluorite-like layer of Sillén oxychlorides.
Apart from excellent oxide ion conductivity, Bi1.9Te0.1LuO4.05Cl also exhibited high electrical conductivity independent of the oxygen partial pressure at 431 °C, which indicated the presence of an electrolyte domain. It also maintained high chemical stability under CO2 flow at 400 °C and ambient air at 600 and 400 °C.
The results of this study demonstrated that triple fluorite-like layers of Sillén oxyhalides can be used to develop high ionic conductors at temperatures below 500 °C, solving a long-standing problem with oxide ion conductors. The high ionic and electrical conductivity and chemical stability of Bi1.9Te0.1LuO4.05Cl may open new avenues for the development of superior oxide ion conductors with a triple fluorite-like layer.
“Solid oxide fuel cells are being widely accepted as the new-age energy source, but the cost is high due to their high operating temperature. Through this research, we have developed new oxide ion conductors, which could significantly lower the operating temperature and reduce their cost,” concludes Yashima.
###
Related Links:
Toward Sustainable Energy Applications with Breakthrough in Proton Conductors |Tokyo Tech News
Shedding Light on Unique Conduction Mechanisms in a New Type of Perovskite Oxide | Tokyo Tech News
About Tokyo Institute of Technology
Tokyo Tech stands at the forefront of research and higher education as the leading university for science and technology in Japan. Tokyo Tech researchers excel in fields ranging from materials science to biology, computer science, and physics. Founded in 1881, Tokyo Tech hosts over 10,000 undergraduate and graduate students per year, who develop into scientific leaders and some of the most sought-after engineers in industry. Embodying the Japanese philosophy of “monotsukuri,” meaning “technical ingenuity and innovation,” the Tokyo Tech community strives to contribute to society through high-impact research.
Journal
Journal of the American Chemical Society
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
High Conductivity and Diffusion Mechanism of Oxide Ions in Triple Fluorite-like Layers of Oxyhalides
Article Publication Date
9-Apr-2024
COI Statement
The authors declare no competing financial interest.