In the field of isotopic geochemistry, precise measurement of magnesium isotope ratios has long been a cornerstone for understanding geological processes and the thermal histories of rocks. Two primary methods have arisen as dominant techniques in this arena: the Sample-Standard-Bracketing (SSB) method and the Double-Spike (DS) method. Both approaches serve to correct instrumental mass bias, a pervasive challenge in isotope ratio measurements, yet they differ fundamentally in procedure, precision, and susceptibility to external variables. Recent advances highlight the DS method’s capability to revolutionize magnesium isotope analysis across multiple geological contexts, promising unprecedented accuracy and reliability in interpreting isotopic variations.
The SSB method operates by analyzing isotopic standards before and after measuring an unknown sample, effectively bracketing the sample to calibrate and correct the instrument’s mass bias. While this approach has been widely adopted due to its conceptual simplicity and effectiveness, its precision is generally constrained to around ±0.06‰ (2 standard deviations), limiting its ability to resolve extremely subtle isotopic fractionations. Another challenge inherent in the SSB approach is the necessity of closely matching the chemical matrix of unknown samples to those of the bracketing standards. Failure to achieve this likeness often introduces systematic discrepancies in the reported δ^26Mg values, complicating inter-laboratory comparisons and interpretations.
In contrast, the Double-Spike method involves spiking the unknown sample with two isotopically enriched magnesium tracers. By carefully measuring the isotope ratios of this spiked mixture, one can mathematically decouple the true isotopic composition of the sample from instrumental mass fractionation as well as from any fractionation induced during chemical processing. The DS technique delivers superior long-term precision of approximately ±0.03‰ (2 standard deviations), effectively doubling the analytical resolution achievable relative to the SSB method. Moreover, it exhibits a markedly reduced sensitivity to sample concentration variations and memory effects within the mass spectrometer, obviating the stringent concentration matching requirements that constrain the SSB method’s efficacy.
One critical procedural consideration within DS methodology lies in the timing of double-spike addition. Traditionally, double spikes are introduced prior to chemical purification, enabling correction of mass-dependent fractionation effects incurred during chromatographic separation. However, this protocol can lead to iterative error accumulation if samples undergo repeated purification. To mitigate this risk, an alternative strategy adds the Mg double spikes post-purification, thereby minimizing cumulative errors and enhancing the robustness of isotope ratio determinations. This refinement reflects an evolving understanding of how best to preserve the integrity of ultra-precise isotopic measurements in complex geological matrices.
The practical importance of these advances extends to the characterization of isotopic fractionation behaviors at elevated temperatures, often exceeding 300 Kelvin, characteristic of many geological environments. It is well-established that isotopic fractionation—the preferential distribution of isotopes between different mineral phases—diminishes as temperature increases, resulting in smaller measurable differences during high-temperature equilibration processes. Thermodynamically, under equilibrium conditions, phases with smaller atomic coordination numbers tend to preferentially incorporate heavier isotopes due to shorter, stronger chemical bonds and higher vibrational energies. This phenomenon manifests in natural settings whereby garnet typically exhibits lighter Mg isotope compositions relative to spinel, which carries heavier Mg isotopes owing to differences in crystal structure and coordination environment.
Utilizing these variations, researchers have proposed novel Mg isotopic thermometry techniques, exploiting the differential fractionations between mineral assemblages to reconstruct the thermal evolution of rocks and tectonic complexes. The success of such isotope-based thermometric tools hinges upon the analytical precision of the isotope measurements themselves. While the SSB method offers adequate precision for examining major rock-forming minerals such as garnet, pyroxene, and olivine, the DS method’s enhanced sensitivity significantly improves the accuracy and reliability of temperature estimations derived from inter-mineral isotope fractionations. This precision gain reduces uncertainties inherent in thermal reconstructions, enabling finer insights into the metamorphic histories recorded in mineral phases.
Beyond thermometry, the DS method’s unparalleled resolution in detecting subtle Mg isotope fractionations lays a strong foundation for its broader applicability across diverse geochemical and cosmochemical systems. The method’s ability to consistently resolve minute isotopic nuances opens pathways for new research directions, from tracing fluid-rock interactions to investigating mantle source heterogeneities and crustal recycling processes. As geoscientists increasingly adopt the DS technique, the potential for uncovering previously obscured geochemical signals becomes a transformative driver for advancing our understanding of Earth’s dynamic interior.
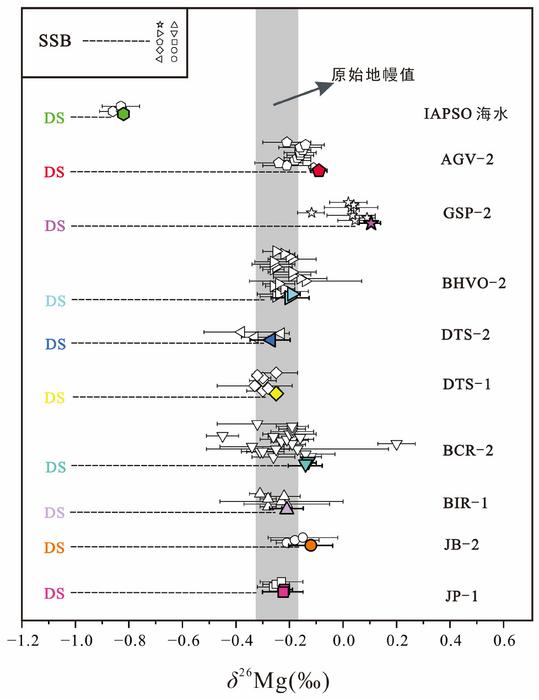
Moreover, expanding the application of the DS method to an extended suite of well-characterized geological standard materials will foster rigorous cross-validation against traditional SSB measurements. This systematic comparative approach is essential to disentangle the fundamental causes of discrepancies observed between the two methodologies. Such efforts not only enhance confidence in inter-laboratory datasets but also catalyze the refinement of analytical protocols, calibration standards, and error models in Mg isotope geochemistry. The resultant convergence of methodology will elevate the overall fidelity of isotopic datasets used in petrological and geochemical modeling.
Critically, the double-spike approach’s robustness against concentration and memory effects also offers logistical advantages in analytical workflows. Laboratories dealing with heterogeneous and complex matrices can achieve consistent isotopic ratios without the stringent sample preparation and matrix matching demanded by SSB protocols. This not only streamlines analytical throughput but also reduces the potential for contamination and sample loss during preparation. Such operational efficiencies are particularly valuable in large-scale studies requiring high sample numbers or when working with precious geological specimens.
In sum, recent developments underscore a pivotal evolution in magnesium isotope analytical capabilities brought forth by the Double-Spike method. The enhanced precision, reduced susceptibility to analytical biases, and procedural flexibility empower geochemists to push the boundaries of resolution in isotopic studies. This uplift permits the elucidation of geological processes at finer scales and challenges longstanding assumptions predicated on data limited by previous methodological constraints. As the DS technique gains wider adoption and further methodological refinements emerge, its impact will resonate across various domains of Earth and planetary sciences.
Consequently, the Double-Spike method occupies a transformative niche within isotope geochemistry, not merely as an alternative analytical approach but as a paradigm shift enabling high-resolution reconstructions of geochemical histories. From probing the thermal evolution of metamorphic terrains to decoding mantle-crust interactions, its implications permeate fundamental questions regarding Earth’s chemical differentiation and tectonic evolution. The ongoing integration of DS methodology with cutting-edge mass spectrometry and sample preparation protocols promises to unveil novel geochemical insights previously inaccessible through conventional frameworks.
To propel progress, future research should prioritize the development of comprehensive standard reference materials calibrated explicitly for DS applications, along with inter-laboratory calibration exercises to ensure methodological consistency. Simultaneously, continued methodological innovation aimed at further minimizing procedural uncertainties will reinforce the technique’s role as a cornerstone analytical tool. Such coordinated advancements will cement the Double-Spike method’s status as an indispensable asset in the quest to decode the intricate isotopic fabric of the Earth.
Ultimately, the DS method exemplifies how methodological ingenuity in isotope geochemistry can unlock new dimensions of understanding Earth’s complex systems, reinforcing the critical link between analytical precision and geological interpretation. As researchers delve deeper into the subtleties of isotopic fractionation signatures harnessed through this technique, the geological sciences stand poised to witness a renaissance in isotopic applications, driven by the pursuit of precision and the quest for clarity in Earth’s dynamic chronicles.
Subject of Research: Magnesium isotope analysis methods in geochemistry
Article Title: Not provided
Web References: http://dx.doi.org/10.1007/s11430-024-1511-6
Image Credits: ©Science China Press
Keywords: Magnesium isotopes, double-spike method, sample-standard-bracketing method, isotope fractionation, geochemical thermometry, mass spectrometry, isotopic precision, geological standards