In the quest to combat Alzheimer’s disease, researchers have turned to the promising potential of novel imaging technologies. A recent study conducted by Park, Kim, and An offers an intriguing lens on this endeavor by focusing on the comparative analysis of ^18F-labeled PET radiopharmaceuticals used in a mouse model of Alzheimer’s disease. The insights obtained from this research not only pave the way for enhanced diagnostic capabilities but also hold implications for therapeutic interventions in a disease that presents profound challenges for patients, caregivers, and healthcare systems worldwide.
Alzheimer’s disease remains one of the leading causes of dementia, afflicting millions globally and contributing to escalating healthcare costs. The pathology of Alzheimer’s is characterized by the accumulation of amyloid plaques and neurofibrillary tangles, both hallmarks that can disrupt neural transmission and lead to cognitive decline. Traditional diagnostic methods often fall short in terms of accuracy and reliability, which can delay intervention and worsen patient outcomes. Hence, innovative approaches, such as those involving advanced radiopharmaceuticals, are essential for early detection and effective management.
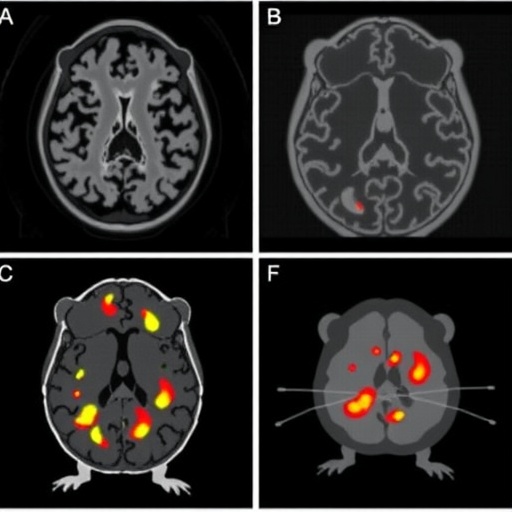
The study investigates the efficacy of various ^18F-labeled radiopharmaceuticals, which are critical for Positron Emission Tomography (PET), an imaging modality that has transformed our understanding of neurological diseases. PET imaging relies on the principles of detecting gamma rays emitted from positron decay of radioactive isotopes that are bound to specific molecules. In Alzheimer’s research, these radiopharmaceuticals can bind to amyloid plaques, allowing for precise imaging and assessment of disease progression in vivo.
What sets this research apart is the comparative nature of the analysis, which systematically evaluates the performance of multiple PET tracers within a controlled mouse model. This is particularly significant as the choice of radiopharmaceutical can greatly influence the sensitivity and specificity of imaging the characteristic pathophysiological features of Alzheimer’s. By examining different compounds, the study provides valuable insights into which radiopharmaceuticals might yield the most informative imaging results, guiding future research and clinical applications.
Throughout their experimentation, Park and colleagues meticulously designed a series of preclinical studies, employing transgenic mouse models engineered to develop Alzheimer’s-like pathology. This approach ensured that the outcomes would closely simulate the human condition, thereby enhancing the relevance and applicability of the findings. The meticulous design and execution of these studies underscore the importance of in vivo models in the leading edge of neuroimaging research.
The researchers did not just stop at imaging; they also delved into the pharmacokinetics and pharmacodynamics of these agents. Understanding how these compounds behave within biological systems is crucial for determining their viability as diagnostic tools. Factors such as the compound’s half-life, clearance rates, and distribution can dramatically influence how well they perform. These parameters allow researchers to predict the optimal time for imaging and how long the compounds remain active within the system.
In bifurcating the data among various parameters, including resolution, brightness, and binding affinity, the study meticulously cataloged the advantages and disadvantages of each radiopharmaceutical. This granularity in analysis facilitates a transparent comparison and aids in decision-making for both clinical and research settings. It emphasizes the necessity for a careful selection process when determining which radiopharmaceuticals offer the most significant benefit in diagnosing Alzheimer’s disease.
Notably, the study’s findings have broader implications beyond technical advancements. By identifying the most effective PET tracers, researchers and clinicians can perhaps improve patient outcomes through earlier and more accurate diagnoses, ultimately allowing for timely therapeutic interventions. This, in turn, could lead to a reduction in the overall burden of care associated with late-stage Alzheimer’s, a condition often characterized by severe cognitive and functional decline.
Additionally, the investigation reflects an ongoing effort to establish a standardized protocol for imaging in Alzheimer’s research, providing researchers across the globe with a robust framework that can be readily adopted. Establishing such consistency is vital for enhancing the reproducibility of research findings, a growing concern in the science community as highlighted by various meta-analyses of preclinical studies.
The emerging landscape of Alzheimer’s diagnostics, aided by advancements in radiopharmaceuticals, embodies a multi-faceted approach. By marrying innovative imaging techniques with a thorough understanding of pathological mechanisms, researchers can forge a pathway toward significant breakthroughs in early diagnostic strategies. This could potentially lead to the surge of novel therapeutic agents that directly target the underlying mechanisms of Alzheimer’s disease, marking a paradigm shift in how we approach neurodegenerative diseases.
In conclusion, the comparative investigation of ^18F-labeled PET radiopharmaceuticals in an Alzheimer’s disease mouse model holds promise for enhancing diagnostic methodologies that are not only reflective of patient needs but also anchored in rigorous scientific validation. The implications extend far beyond the laboratory, impacting clinical practice, patient care, and ultimately enhancing the quality of life for individuals battling Alzheimer’s. As we continue to seek solutions to this daunting disease, studies like this stand as beacons of hope, guiding us toward a future where early detection and targeted therapies become the standard in care.
Subject of Research: Comparisons of ^18F-labeled PET radiopharmaceuticals in Alzheimer’s disease models.
Article Title: Comparative study of ^18F-labeled PET radiopharmaceuticals in an Alzheimer’s disease mouse model.
Article References: Park, BN., Kim, SM. & An, YS. Comparative study of ^18F-labeled PET radiopharmaceuticals in an Alzheimer’s disease mouse model. BMC Neurosci 26, 55 (2025). https://doi.org/10.1186/s12868-025-00978-0
Image Credits: AI Generated
DOI: https://doi.org/10.1186/s12868-025-00978-0
Keywords: Alzheimer’s disease, PET radiopharmaceuticals, imaging techniques, diagnostics, neurodegeneration, pharmacokinetics, animal model, amyloid plaques.