In a groundbreaking study that could reshape our understanding of gestational diabetes mellitus (GDM) and its implications for fetal development, researchers have uncovered a significant role played by the gene CHAF1A in preadipocyte differentiation. This discovery provides deeper insights into how macrosomia, a condition where infants are born with excessive weight, is influenced during pregnancy. Researchers Xia, Xu, and Zhang, along with their team, have meticulously investigated the intricacies of how CHAF1A functions at the cellular level, thereby unveiling potential pathways that could be targeted to mitigate risks associated with GDM.
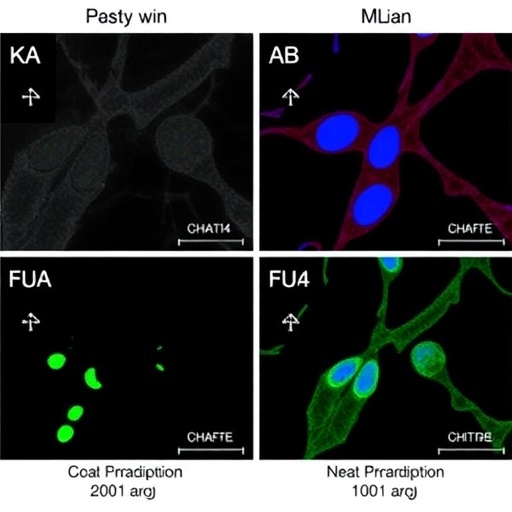
CHAF1A, or Chromatin-Accessing Factor 1A, is a component of the chromatin remodeling complex, which is essential for DNA replication and transcription. The study highlights that CHAF1A not only facilitates the structural integrity of chromatin but also plays a pivotal role in the differentiation of preadipocytes—precursor cells that eventually develop into adipocytes, the cells responsible for storing fat in the body. Through a series of elegant experiments, the researchers demonstrated that an elevated expression of CHAF1A in preadipocytes led to an increased rate of differentiation, suggesting a direct link between gene expression modulation and cellular maturation in the context of gestational diabetes.
Gestational diabetes is characterized by glucose intolerance that arises during pregnancy and poses significant health risks to both the mother and the fetus. The implications of excessive maternal glucose levels can manifest in various ways, with macrosomia being one of the most concerning outcomes. Babies born with macrosomia are at a higher risk of complications during delivery and may face long-term health issues, including obesity and metabolic disorders later in life. The findings of this study encourage further exploration of the mechanisms by which maternal gene expression impacts fetal growth trajectories and adipose tissue development.
The researchers conducted a range of in vitro and in vivo experiments to elucidate the role of CHAF1A in GDM. In their in vitro analyses, the differentiation of preadipocytes was significantly enhanced in the presence of high glucose concentrations, mirroring the metabolic environment of a pregnant woman suffering from gestational diabetes. Notably, when CHAF1A was selectively knocked down, the preadipocyte differentiation process was considerably impaired, underscoring the gene’s indispensable role in this process. These findings not only illuminate the biological functions of CHAF1A but also raise critical questions about its potential as a therapeutic target in managing gestational diabetes and preventing macrosomia.
Given the escalating rates of gestational diabetes globally, the implications of such research could be profound. Understanding the mechanistic pathways involving CHAF1A could pave the way for novel interventions aimed at optimizing maternal health during pregnancy. For instance, therapeutic strategies designed to modulate CHAF1A expression may help manage glucose levels or fatty tissue accumulation in preadipocytes, ultimately reducing the incidence of macrosomia. This could lead to a far-reaching impact not just on maternal and neonatal health, but also on public health systems grappling with the long-term consequences of metabolic diseases stemming from early developmental conditions.
The study also presents a compelling case for further research into other chromatin remodeling factors that might contribute to metabolic dysregulation during pregnancy. What remains to be explored is whether interventions targeting CHAF1A or similar genes could be effectively employed in clinical settings. The effectiveness and safety of such strategies would need to be rigorously evaluated through clinical trials. Understanding the timeline of CHAF1A expression during pregnancy, as well as its interaction with other metabolic pathways, will be essential for developing these interventions.
In light of current healthcare challenges, the need for innovative solutions to combat the rising prevalence of gestational diabetes cannot be overstated. The study’s findings serve as a clarion call for medical researchers and practitioners to reconsider traditional approaches to managing GDM, placing greater emphasis on the genetic and molecular underpinnings of the condition. As we gain deeper insights into the roles played by specific genes like CHAF1A, the potential for personalized medicine approaches tailored to individuals at high risk for gestational diabetes becomes increasingly tangible.
Moreover, the implications of this research extend beyond immediate treatment strategies. By elucidating the cellular and molecular basis for macrosomia linked to gestational diabetes, there is an opportunity to inform future prenatal care practices. This could promote healthier gestational weight gain in expectant mothers, reduce metabolic risks, and enhance the overall well-being of mothers and their babies. The prospect of achieving better health outcomes calls for a concerted effort among endocrinologists, obstetricians, and geneticists to collaborate more closely in creating comprehensive care plans for pregnant women.
As researchers continue to delve into the complexities of gestational diabetes, the exploration of CHAF1A is likely to be a pivotal point of focus. The challenge lies not only in understanding the implications of the findings, but also in translating them into actionable healthcare strategies. It might mean heralding a new era in the fight against gestational diabetes, framed by a richer understanding of genetic factors influencing pregnancy.
The findings laid out in the study of Xia, Xu, and Zhang foreshadow a future where gestational diabetes can be tackled with targeted genetic interventions, potentially reducing the burden of macrosomia. It emphasizes the importance of integrating genetic research into clinical practice, ultimately leading to improved health outcomes. As the inquiry continues, the trajectory of maternal-fetal health could be poised for transformation, informing fresh pathways toward prevention and treatment in the realm of endocrine dysfunction during pregnancy.
In conclusion, the research encompassing the role of CHAF1A in preadipocyte differentiation and its implications for macrosomia in gestational diabetes enceinte a unique blend of genetics and maternal health. As the scientific community stands on the brink of new discoveries that could redefine the landscape of prenatal care, the promise of CHAF1A as a prospective therapeutic target shines brightly in the evolving narrative of gestational diabetes management. The ripple effects of such monumental insights could extend far beyond the clinic, reaching into public health policies and possibly leading to a paradigm shift in maternal healthcare.
As we eagerly await the next wave of research emerging from these findings, the path forward involves a mix of scientific rigor, exploratory risk-taking, and a commitment to improving outcomes for pregnant women and their children. The integration of genetic understanding into everyday clinical practice represents not just an opportunity, but a necessity in effectively addressing the challenges posed by gestational diabetes in our modern healthcare landscape.
Subject of Research: The role of CHAF1A in preadipocyte differentiation and its connection to macrosomia in gestational diabetes mellitus.
Article Title: CHAF1A Promotes Preadipocyte Differentiation and Contributes to Macrosomia in Gestational Diabetes Mellitus.
Article References: Xia, D., Xu, X., Zhang, Y. et al. CHAF1A Promotes Preadipocyte Differentiation and Contributes to Macrosomia in Gestational Diabetes Mellitus. Reprod. Sci. (2025). https://doi.org/10.1007/s43032-025-01946-z
Image Credits: AI Generated
DOI: 10.1007/s43032-025-01946-z
Keywords: CHAF1A, preadipocyte differentiation, gestational diabetes mellitus, macrosomia, maternal health, genetics, chromatin remodeling