In a groundbreaking study published in BMC Cancer, researchers have unveiled the potent anticancer properties of cephalomannine, a compound showing remarkable efficacy against pleural mesothelioma (PM), a notoriously aggressive and treatment-resistant form of cancer. This research not only highlights the therapeutic potential of cephalomannine but also elucidates the cellular mechanisms underpinning its anticancer activity, offering renewed hope for patients suffering from this devastating disease.
Pleural mesothelioma, primarily caused by asbestos exposure, has long challenged oncologists due to its poor prognosis and resistance to conventional therapies. The urgent need for more effective treatment strategies has driven scientists to explore novel agents capable of halting or reversing tumor progression. Cephalomannine, a taxane compound structurally related to paclitaxel, emerged as a standout candidate during an extensive drug screening process aimed at identifying molecules with high activity against mesothelioma cells.
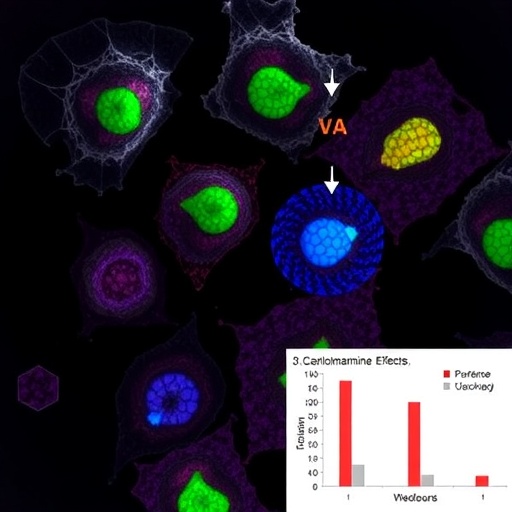
The research team conducted rigorous in vivo experiments using NOD-SCID mice implanted with human PM xenografts to assess the therapeutic efficacy of cephalomannine in a living system. Mice treated with cephalomannine at a dosage of 2 mg/kg body weight exhibited a significantly prolonged median overall survival (OS) of 75 days compared to only 55 days in the vehicle-treated control group. This statistically significant improvement underscores cephalomannine’s ability to meaningfully extend survival in an aggressive cancer model.
In addition to animal studies, the investigators delved into the molecular and cellular impacts of cephalomannine in vitro, using two different cell lines: REN, a human pleural mesothelioma-derived line, and MeT-5A, a non-malignant mesothelial cell line serving as a control. This dual-cell approach allowed for a nuanced understanding of cephalomannine’s selective effects on cancerous versus normal cells, critical for evaluating therapeutic safety and specificity.
One of the key revelations from the in vitro experiments was the alteration in cellular metabolism induced by cephalomannine. The compound markedly decreased the oxygen consumption rate (OCR) in REN cells, indicating a disruption in mitochondrial respiration. Such an effect suggests that cephalomannine impairs the energy production pathways cancer cells rely on, potentially triggering metabolic stress and sensitizing tumors to apoptotic signals.
Complementing the metabolic findings, cephalomannine treatment led to a significant increase in the production of reactive oxygen species (ROS) within mesothelioma cells. Elevated ROS levels, while damaging at high concentrations, can act as a double-edged sword in cancer cells by inducing oxidative stress that contributes to cell death. This pro-oxidative effect of cephalomannine provides a mechanistic insight into its cytotoxicity.
Further molecular analyses revealed that cephalomannine modulates key regulators of apoptosis, the programmed cell death pathway often dysregulated in cancer. Specifically, cephalomannine increased the protein expression of BAX, a pro-apoptotic molecule, while decreasing Bcl-2, an anti-apoptotic counterpart, in the REN cell line. This shift in the BAX/Bcl-2 ratio favors apoptotic signaling, promoting the elimination of malignant cells.
Interestingly, despite these pro-death effects, cephalomannine did not activate inflammatory pathways typically associated with cancer cell responses. The levels of interleukin-18 (IL-18), a cytokine marker for inflammasome activation, remained unchanged, suggesting that cephalomannine’s anticancer activity is primarily driven through apoptosis rather than inflammation. This selective mechanism may reduce potential side effects linked to inflammatory responses.
While fludarabine and crenolanib, two other agents tested in parallel, failed to demonstrate significant efficacy in the in vivo mesothelioma model, cephalomannine clearly stood out as a potent therapeutic candidate. These comparative results highlight the importance of thorough preclinical screening to identify truly effective anti-cancer agents.
The collective findings from this study illuminate cephalomannine as a multifaceted anticancer compound that disrupts mitochondrial function, induces oxidative stress, and triggers apoptosis selectively in mesothelioma cells. This multifactorial mechanism makes it a promising candidate for further development, either as a monotherapy or in combination with existing chemotherapeutics to overcome resistance.
Moreover, this work opens new avenues for investigating novel molecular targets within the metabolic and apoptotic pathways modulated by cephalomannine. Understanding these pathways in greater detail could lead to the design of even more effective derivatives or combinatorial regimens tailored for mesothelioma treatment.
Given the limited treatment options currently available for pleural mesothelioma patients, these findings hold significant clinical potential. If future clinical trials confirm cephalomannine’s safety and efficacy in humans, it could revolutionize the standard of care and improve long-term outcomes for individuals afflicted with this aggressive cancer.
In sum, this study not only brings cephalomannine to the forefront of mesothelioma research but also exemplifies the power of integrating in vivo survival data with detailed mechanistic insights. Such an approach enhances our understanding of cancer biology and accelerates the translation of laboratory discoveries into tangible therapeutic advancements.
Researchers are now poised to embark on the next stage of investigations, including dose optimization, toxicity profiling, and clinical trials, to fully harness cephalomannine’s anticancer potential. The ongoing quest to outsmart mesothelioma has gained a formidable new contender, signaled by the promise of this remarkable compound.
This pioneering work stands as a testament to the relentless pursuit of innovative solutions in cancer therapy, demonstrating that even well-known molecules can reveal unexpected efficacies in challenging malignancies like pleural mesothelioma.
As the scientific community rallies around these findings, the hope for effective, targeted treatments for mesothelioma soars, offering patients and clinicians new reasons to look forward to a future with better therapeutic options.
Subject of Research: The anticancer effects and mechanisms of action of cephalomannine in pleural mesothelioma cells.
Article Title: Cephalomannine as in vitro and in vivo anticancer agent in mesothelioma cells: mechanisms of action
Article References:
Morani, F., Dell’Anno, I., Daga, A. et al. Cephalomannine as in vitro and in vivo anticancer agent in mesothelioma cells: mechanisms of action. BMC Cancer 25, 1625 (2025). https://doi.org/10.1186/s12885-025-14902-6
Image Credits: Scienmag.com