A groundbreaking discovery by an international team of scientists from the United Kingdom and South Africa has unveiled an unexpected role for the widely-used antibiotic ciprofloxacin, potentially revolutionizing treatments for high blood pressure and cardiovascular diseases. This innovative research elucidates a novel mechanism by which ciprofloxacin inhibits the Angiotensin-Converting Enzyme (ACE), an enzyme crucially involved in the regulation of blood pressure. By revealing a previously unrecognized binding site distinct from conventional ACE inhibitors, this discovery promises to spur the development of a new generation of hypertensive drugs with enhanced specificity and reduced adverse effects.
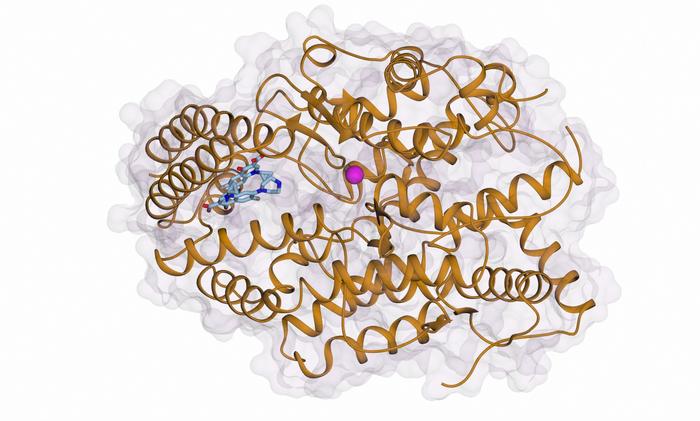
The researchers, led by Professor Ravi Acharya from the University of Bath and Professor Ed Sturrock from the Institute of Infectious Disease and Molecular Medicine at the University of Cape Town, have demonstrated that ciprofloxacin interacts with ACE in an unconventional manner. Unlike existing drugs that target the zinc-containing catalytic pocket within ACE, ciprofloxacin binds to an allosteric site located on the C-domain of the enzyme. This allosteric site is spatially distinct from the active site, and the binding of ciprofloxacin here effectively blocks the conversion of angiotensin I to angiotensin II without compromising the enzyme’s other physiological functions.
ACE plays a pivotal role in the renin-angiotensin system by elevating blood pressure through the conversion of angiotensin I, an inactive precursor, into angiotensin II, a potent vasoconstrictor responsible for narrowing blood vessels. While ACE inhibitors have been the cornerstone of antihypertensive therapy for decades, their mechanism of action involves binding to the active site of ACE, which also contributes to off-target effects. These effects manifest clinically as troublesome side effects, including a persistent dry cough and angioedema, limiting patient compliance and therapeutic efficacy.
The dual-domain architecture of ACE, encapsulating both the N-terminal and C-terminal domains with distinct active sites, has long posed challenges for the design of selective inhibitors. Current medications indiscriminately inhibit both domains, inadvertently influencing non-blood-pressure-related physiological processes such as renal function, reproductive health, and immune responses. The promiscuity of ACE inhibitors has thus been a significant hurdle in pharmaceutical development, motivating the search for selective modulators that can fine-tune enzyme activity more precisely.
In this study, published in ACS Bio & Med Chem Au, the team employed advanced X-ray crystallography techniques to resolve the three-dimensional structure of ACE in complex with ciprofloxacin. Their structural data reveal that ciprofloxacin docks into a previously uncharacterized allosteric exosite located on the C-domain. This binding event induces a conformational change that occludes the substrate angiotensin I from accessing the active site, effectively suppressing ACE activity related to blood pressure without interfering with the enzyme’s multifaceted physiological roles.
The allosteric inhibition mechanism identified here represents a paradigm shift in ACE drug design. By targeting an exosite remote from the catalytic zinc ion, allosteric inhibitors like ciprofloxacin analogues could offer significant therapeutic advantages. These include increased selectivity, reduced adverse effects, and the preservation of ACE functions outside blood pressure regulation. This discovery opens avenues for the development of tailor-made ACE inhibitors that maintain cardiovascular efficacy while minimizing systemic toxicity and patient discomfort associated with current treatments.
Although ciprofloxacin itself binds relatively weakly to the ACE allosteric site and is unlikely to function as a standalone antihypertensive medication, its chemical scaffold provides a valuable template for medicinal chemistry efforts. The research team envisions that optimizing ciprofloxacin derivatives could enhance binding affinity and specificity, culminating in a new class of ACE inhibitors engineered to exploit the allosteric mechanism. Such drugs would not only improve patient outcomes but also expand the pharmacological toolkit available to clinicians managing hypertension and related cardiovascular conditions.
The collaborative nature of this research, supported by UKRI-BBSRC and involving a confluence of expertise in enzymology, structural biology, and pharmacology, underscores the importance of interdisciplinary approaches in addressing complex biomedical challenges. Dr. Vinasha Ramasamy and Professor Ed Sturrock contributed essential enzymatic kinetics analyses, while Dr. Kyle Gregory and Professor Ravi Acharya’s team meticulously determined the ACE-ciprofloxacin complex structure. Their combined efforts have illuminated a novel path for antihypertensive drug discovery that merges structural insight with functional biochemistry.
Looking forward, the team is poised to screen and synthesize various chemical analogues of ciprofloxacin to refine their interaction with ACE’s allosteric site. By leveraging structure-guided drug design and high-throughput screening, future compounds can be tailored to maximize therapeutic efficacy while minimizing side effects. This approach holds promise for addressing the global burden of hypertension, which affects approximately one in three adults in the UK, and for whom ACE inhibitors remain a critical component of treatment despite their limitations.
Beyond potential pharmaceutical applications, the findings have broader implications for understanding enzyme regulation via allosteric modulation. The ability to selectively inhibit a multi-functional enzyme like ACE through distal binding sites exemplifies an elegant biological control mechanism and inspires analogous strategies in drug discovery targeting other clinically relevant enzymes. This work exemplifies how repurposing known drugs can reveal latent activities, thereby accelerating the path from bench to bedside.
In conclusion, this seminal study reframes our understanding of ACE inhibition by unveiling ciprofloxacin’s unexpected capacity to engage an allosteric exosite on the ACE C-domain, impeding angiotensin I conversion without wholesale enzymatic blockade. Such insights herald a transformative approach in the design of future antihypertensive agents that combine precision, safety, and efficacy. As the global incidence of hypertension escalates, innovations like this are indispensable in refining therapeutic regimens and improving patient quality of life.
Subject of Research: Not applicable
Article Title: Ciprofloxacin Inhibits Angiotensin I-Converting Enzyme (ACE) Activity by Binding at the Exosite, Distal to the Catalytic Pocket
News Publication Date: 9-Jun-2025
Web References:
https://pubs.acs.org/doi/10.1021/acsbiomedchemau.5c00089
References:
Acharya, R. et al. (2025) ‘Ciprofloxacin Inhibits Angiotensin I-Converting Enzyme (ACE) Activity by Binding at the Exosite, Distal to the Catalytic Pocket’, ACS Bio & Med Chem Au, DOI: 10.1021/acsbiomedchemau.5c00089.
Image Credits: Professor Ravi Acharya, University of Bath
Keywords:
Drug discovery, Drug design, Drug targets, Drug candidates, Enzymatic activity, Enzyme inhibitors, Structural biology, Biomolecular structure, Tertiary structure, Activation loops, Binding pockets, Antihypertensive activity, Vasopressors