“[…] we have characterized a pair of compounds that impact multiple processes that are relevant to cancer cell proliferation but also, and possibly more importantly, to metastasis […].”

Credit: 2024 Shkreta et al.
“[…] we have characterized a pair of compounds that impact multiple processes that are relevant to cancer cell proliferation but also, and possibly more importantly, to metastasis […].”
BUFFALO, NY- May 20, 2024 – A new research paper was published in Oncotarget’s Volume 15 on May 16, 2024, entitled, “The anticancer potential of the CLK kinases inhibitors 1C8 and GPS167 revealed by their impact on the epithelial-mesenchymal transition and the antiviral immune response.”
The diheteroarylamide-based compound 1C8 and the aminothiazole carboxamide-related compound GPS167 inhibit the CLK kinases, and affect the proliferation of a broad range of cancer cell lines. A chemogenomic screen previously performed with GPS167 revealed that the depletion of components associated with mitotic spindle assembly altered sensitivity to GPS167.
In this new study, researchers Lulzim Shkreta, Johanne Toutant, Aurélie Delannoy, David Durantel, Anna Salvetti, Sophie Ehresmann, Martin Sauvageau, Julien A. Delbrouck, Alice Gravel-Trudeau, Christian Comeau, Caroline Huard, Jasmin Coulombe-Huntington, Mike Tyers, David Grierson, Pierre-Luc Boudreault, and Benoit Chabot from Université de Sherbrooke, Université de Lyon, Institut de recherches cliniques de Montréal, Université de Montréal, and University of British Columbia a similar screen performed with 1C8 also established the impact of components involved in mitotic spindle assembly.
“Accordingly, transcriptome analyses of cells treated with 1C8 and GPS167 indicated that the expression and RNA splicing of transcripts encoding mitotic spindle assembly components were affected.”
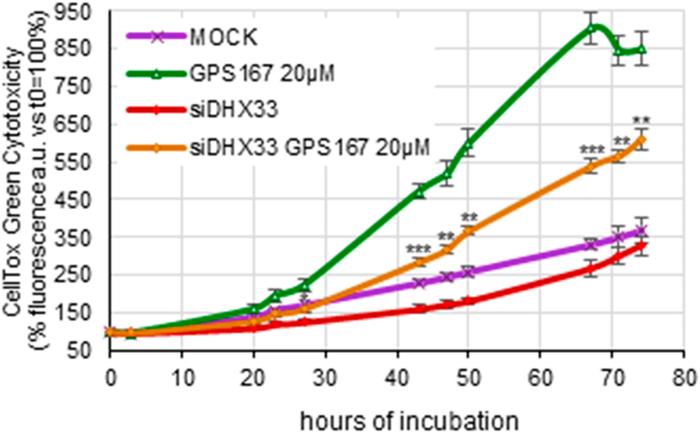
The functional relevance of the microtubule connection was confirmed by showing that subtoxic concentrations of drugs affecting mitotic spindle assembly increased sensitivity to GPS167. 1C8 and GPS167 impacted the expression and splicing of transcripts in pathways relevant to tumor progression, including MYC targets and the epithelial mesenchymal transition (EMT). Finally, 1C8 and GPS167 altered the expression and alternative splicing of transcripts involved in the antiviral immune response. Consistent with this observation, depleting the double-stranded RNA sensor DHX33 suppressed GPS167-mediated cytotoxicity on HCT116 cells.
“Our study uncovered molecular mechanisms through which 1C8 and GPS167 affect cancer cell proliferation as well as processes critical for metastasis.”
Continue reading: DOI: https://doi.org/10.18632/oncotarget.28585
Correspondence to: Benoit Chabot
Email: benoit.chabot@usherbrooke.ca
Keywords: CLK kinases inhibitors, EMT, antiviral immune response, microtubules, metastasis
Click here to sign up for free Altmetric alerts about this article.
About Oncotarget: Oncotarget (a primarily oncology-focused, peer-reviewed, open access journal) aims to maximize research impact through insightful peer-review; eliminate borders between specialties by linking different fields of oncology, cancer research and biomedical sciences; and foster application of basic and clinical science.
Oncotarget is indexed and archived by PubMed/Medline, PubMed Central, Scopus, EMBASE, META (Chan Zuckerberg Initiative) (2018-2022), and Dimensions (Digital Science).
To learn more about Oncotarget, visit Oncotarget.com and connect with us on social media:
- X, formerly Twitter
- YouTube
- Spotify, and available wherever you listen to podcasts
Click here to subscribe to Oncotarget publication updates.
For media inquiries, please contact media@impactjournals.com.
Oncotarget Journal Office
6666 East Quaker Street., Suite 1A
Orchard Park, NY 14127
Phone: 1-800-922-0957 (option 2)
###
Journal
Oncotarget
Method of Research
Experimental study
Subject of Research
People
Article Title
The anticancer potential of the CLK kinases inhibitors 1C8 and GPS167 revealed by their impact on the epithelial-mesenchymal transition and the antiviral immune response
Article Publication Date
16-May-2024