A groundbreaking study recently unveiled by researchers at Tsinghua University and Changping Laboratory illuminates a sophisticated mechanism by which the immune system sustains durable defensive memory in the lungs following viral infections. This research, published in Science China Life Sciences, delves into the dynamic behavior of tissue-resident memory T cells (TRM cells) in the respiratory tract and reveals a previously underappreciated migration pattern that balances local immunity with systemic preparedness.
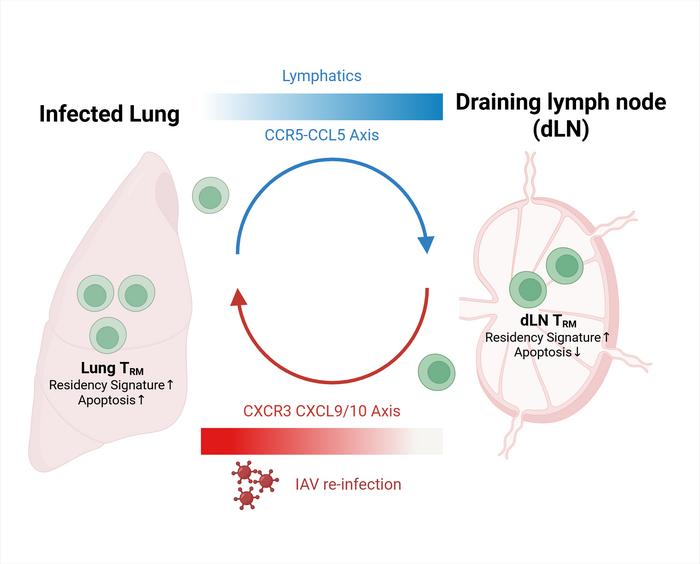
Tissue-resident memory T cells serve as frontline sentinels to swiftly identify and respond to viral pathogens in barrier tissues such as the lungs. Traditionally, TRM cells were thought to be strictly localized, maintaining protection at the site of initial infection without migrating to distant lymphoid tissues. However, the current study challenges this dogma by demonstrating that lung TRM cells can migrate retrogradely—that is, from the lung back to the lung-draining lymph nodes (dLN)—thereby creating a reservoir that sustains immune vigilance beyond the infected tissue.
Using an array of sophisticated single-cell tracking methodologies in murine models infected with influenza A virus (IAV), the researchers tracked lung TRM cells over time. These techniques allowed for precise lineage tracing and gene expression profiling of individual T cells, providing unprecedented resolution of their migratory patterns and functional states. Remarkably, the molecular signatures of TRM cells found in the lung-draining lymph nodes closely matched those derived from the lung tissue, indicative of retrograde migration rather than independent local differentiation.
At the molecular level, the chemokine receptor CCR5 emerged as a pivotal factor orchestrating this trafficking. CCR5 interacts with its ligand, CCL5, to guide the movement of TRM cells from the lung environment toward the lymph nodes. Functional blockade experiments using the CCR5 antagonist Maraviroc resulted in a pronounced reduction of TRM cells in the draining lymph nodes, confirming the receptor’s role in this directional migration. Importantly, CCR5 inhibition did not affect TRM cell numbers within the lungs, isolating the receptor’s effect to the lung-to-lymph node migratory axis.
Inside the draining lymph nodes, TRM cells exhibit reduced apoptotic rates compared to their counterparts in lung tissue, suggesting these lymphoid niches provide survival signals that maintain a stable memory pool. The crosstalk between lung and lymph node TRM cells forms a dynamic maintenance loop: lung-derived TRM cells retrograde migrate and persist in the dLN with preserved transcriptional identity, while upon secondary viral challenge, these dLN-resident TRM cells can re-enter the lung tissue and differentiate back into resident memory cells, reinforcing localized immunity.
This novel loop of retrograde migration and repercussion is of critical importance, especially in the context of recurrent infections such as influenza where rapid reactivation of protective immunity can substantially minimize disease severity. It signifies that antiviral memory is not confined to a single tissue compartment but is actively managed through inter-organ cellular traffic that balances frontline defense with the preservation of long-term memory.
Furthermore, the implications of these findings extend to vaccine development strategies. Traditional vaccination approaches primarily target systemic immunity, often neglecting the tissue-resident lymphocyte populations crucial for prompt local response. By understanding the molecular underpinnings and maintenance dynamics of TRM cells, especially the CCR5-driven migratory circuit, next-generation vaccines can be designed to strategically harness or amplify this pathway, leading to more durable and potent immunity in mucosal tissues like the lungs.
The study also opens exciting avenues for therapeutic intervention. Considering that CCR5 is already a druggable target—with existing FDA-approved antagonists used in other disease contexts such as HIV infection—pharmacological modulation of this pathway could enhance or dampen tissue-specific immunity as clinically warranted. This could be particularly beneficial in respiratory diseases where excessive inflammation or inadequate immune surveillance plays a role.
Importantly, the researchers emphasize that the preservation of TRM cells through retrograde migration does not compromise their effector functions. Single-cell transcriptomic analyses revealed that these cells retain their capacity for rapid cytokine production and cytotoxic activity, even after relocation and persistence within lymph nodes. This retention of functional competency underscores the evolutionary advantage of this bidirectional migration loop: sustained readiness without functional exhaustion.
From a broader immunological perspective, these findings challenge the long-held notion that memory T cells in peripheral tissues exist in isolation. Instead, they reveal complex, physiologically relevant migratory networks that ensure systemic coordination and maintenance of immune memory. Such mechanisms likely apply beyond the lungs to other mucosal and barrier tissues, suggesting a universal principle of tissue immunity.
Corresponding author Hai Qi stated, “Our study reveals a highly dynamic yet finely tuned maintenance loop between the lung and its draining lymph nodes. This extends the conceptual framework of tissue-resident immunity and offers concrete molecular targets to enhance long-term protection, particularly against respiratory viruses. Future work will assess whether similar circuits exist in human tissues and how they can be leveraged clinically.”
Institutions such as Tsinghua University, a global leader in scientific innovation, and the biomedical-focused Changping Laboratory have contributed significantly to this advancement by integrating cutting-edge immunological tools with translational research perspectives. Their collaborative approach underscores the importance of interdisciplinary methods in unraveling complex immune dynamics.
As respiratory pathogens continue to challenge public health worldwide, particularly amid recurring pandemics, insights into the persistence and mobilization of TRM cells provide a promising foothold for novel interventions. This study not only enriches fundamental immunology but also inspires hope for improved clinical outcomes through vaccine refinement and novel immunomodulatory approaches.
In summary, this research offers compelling evidence that lung tissue-resident memory T cells do not function in permanent isolation but engage in a regulated migratory circuit facilitated by the CCR5–CCL5 axis. By migrating retrogradely to the lymph nodes and maintaining viability and identity there, these cells contribute to a robust and adaptable immune memory system poised to defend the respiratory tract upon reinfection. Such findings redefine our understanding of immune memory compartmentalization and open a new frontier in respiratory immunology.
Subject of Research: Tissue-resident memory T cell dynamics in lung and lymph node interaction post-influenza infection.
Article Title: Not provided.
News Publication Date: Not provided.
Web References: http://dx.doi.org/10.1007/s11427-024-2920-y
References: Not provided.
Image Credits: ©Science China Press
Keywords: tissue-resident memory T cells, TRM, retrograde migration, CCR5, CCL5, influenza A virus, lung immunity, lymph nodes, immune memory maintenance, Maraviroc, respiratory tract immunity