Malignant melanoma remains one of the deadliest forms of skin cancer, notorious for its aggressive nature and resilience against current therapeutic interventions. A critical barrier to effective treatment lies within its complex tumor microenvironment (TME), a heterogeneous milieu comprising immune and stromal cells, extracellular matrix components, and diverse signaling molecules. New research emerging from Central South University has shed light on a pivotal signaling axis involving adenosine phosphate molecules, revealing its profound impact on TME dynamics and immunity. This discovery critically advances our understanding of melanoma biology and uncovers promising avenues for enhancing immunotherapy strategies.
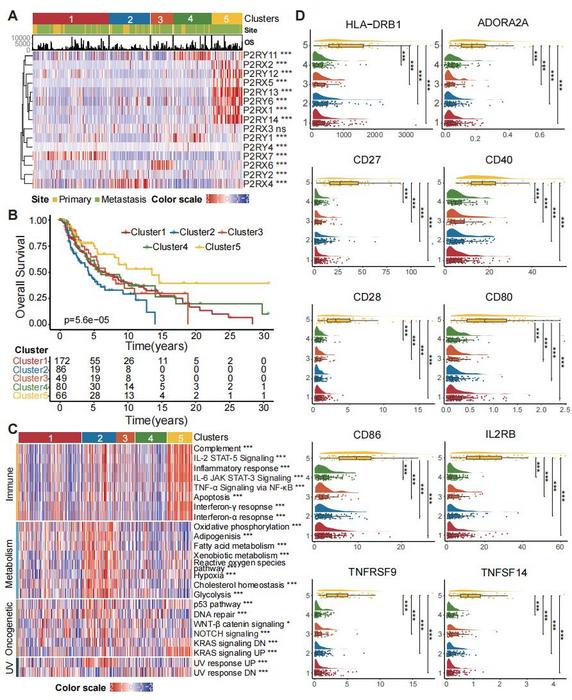
At the heart of this breakthrough is the role of purinergic signaling, mediated by purinergic P2 receptors (P2Rs), which respond to extracellular adenosine nucleotides such as ATP and ADP. These molecules act as danger signals within the tumor microenvironment, orchestrating immune responses and influencing metabolic reprogramming. The research team utilized comprehensive multi-omics analyses, integrating transcriptomic, proteomic, and epigenomic data, to categorize melanoma tumors into five distinct adenosine phosphate signaling subtypes. These subtypes cluster into two overarching metaprograms: a metabolic group characterized by hypoxia and altered bioenergetics, and an inflammatory group with heightened immune activation.
Among the identified subtypes, Subtype 5 emerged as particularly noteworthy due to its elevated expression of P2RX1, P2RY12, and P2RY13 receptors. This "high APsig" cluster exhibited robust inflammatory signatures associated with interferon-gamma (IFN-γ) signaling pathways, increased antigen processing capabilities, and heightened immune cell infiltration. Conversely, Subtype 2, marked by low APsig expression, displayed metabolic rewiring consistent with hypoxic adaptations, suggesting a distinct TME landscape less conducive to effective immune surveillance.
To translate these molecular insights into prognostic and therapeutic tools, the researchers introduced the Adenosine Phosphate Signaling Model (APsig), designed to quantify the extent of adenosine phosphate signaling activity within tumors. By applying APsig scoring across nine independent public melanoma cohorts totaling over 1,000 patients, they discovered a compelling correlation between elevated APsig levels and prolonged overall survival. Remarkably, in patient subsets undergoing treatment with immune checkpoint inhibitors targeting PD-1 and PD-L1, those classified as high APsig responders demonstrated substantially higher remission rates — up to 50% better than their low APsig counterparts.
Delving deeper into cellular mechanisms, single-cell RNA sequencing provided unprecedented resolution of APsig activity within the TME. The data indicated that myeloid lineage cells, including macrophages and conventional dendritic cell subtype 1 (cDC1), exhibit pronounced activation of this signaling axis. These cells appear instrumental in antigen presentation through enhanced expression of major histocompatibility complex (MHC) class I and II molecules, effectively priming cytotoxic CD8+ T cells and orchestrating adaptive immune responses. Intriguingly, spatial transcriptomic mapping further illuminated that regions with high APsig correspond to immune-enriched niches particularly localized at tumor-stroma interfaces—critical zones for immune-tumor cell interaction and immune surveillance.
A notable aspect of this study is the inverse relationship observed between APsig and immunosuppressive elements within the melanoma microenvironment. High APsig tumors were characterized by a depletion of M2-type macrophages, known for their roles in immune suppression and tissue remodeling, as well as reduced presence of resting natural killer (NK) cells that lack cytotoxic activity. Concurrently, these tumors exhibited increased infiltration of activated cytotoxic T lymphocytes and greater diversity in T cell receptor repertoires, underscoring a "hot" tumor phenotype indicative of robust antitumor immunity.
These revelations position APsig not only as a window into melanoma biology but as a powerful biomarker with dual prognostic and predictive capacities. Its value surpasses traditional markers such as tumor mutation burden (TMB) and PD-L1 expression, offering higher sensitivity and specificity in anticipating responses to immune checkpoint therapies. This advancement could revolutionize patient stratification in clinical settings, optimizing therapeutic decisions and minimizing exposure to ineffective treatments.
Moreover, this body of work introduces new therapeutic vistas that exploit adenosine phosphate signaling pathways. By pharmacologically enhancing or mimicking APsig activity, clinicians may potentiate the efficacy of established immune checkpoint inhibitors. The integration of purinergic signaling modulators with immunotherapy could synergistically amplify immune activation, overcoming tumor-mediated immune evasion and metabolic constraints that undermine T cell function.
Looking beyond melanoma, the research team envisions broad applications of APsig across various solid tumor types, where similar TME dynamics prevail. Ongoing clinical trials aim to validate APsig as a universal biomarker and evaluate its utility in guiding combination therapies tailored to individual tumor immunometabolic profiles. The prospect of harnessing adenosine phosphate signaling to reshape tumor ecosystems marks a paradigm shift in oncology, blurring the line between metabolism and immunity in cancer therapy.
Central South University’s groundbreaking study exemplifies the power of cutting-edge multi-omic approaches coupled with spatial and single-cell technologies to decode the intricacies of tumor immunology. It also underscores the critical role of stable yet dynamic purinergic signaling networks in modulating the balance between immune activation and suppression. As immunotherapy continues to transform cancer care, biomarkers like APsig promise to sharpen the precision of this revolution, enabling personalized interventions that maximize clinical benefit.
In conclusion, the intricate dance between adenosine phosphate signals and immune cell populations within the melanoma microenvironment reveals a finely-tuned regulatory axis vital for antitumor immunity. Through the identification and application of APsig, researchers have illuminated new directions for prognosis, treatment prediction, and innovative therapeutic combinations. This landmark work not only elevates our conceptual framework of TME biology but also fast-tracks the translation of novel scientific insights into tangible clinical impact, potentially improving outcomes for melanoma patients worldwide.
Subject of Research: The role of adenosine phosphate signaling mediated by purinergic P2 receptors in melanoma tumor microenvironment and its impact on antitumor immunity and immunotherapy outcomes.
Article Title: Activation of Adenosine Phosphate Signaling Promotes Antitumor Immunity in Tumor Microenvironment and Facilitate Immunotherapy
News Publication Date: 24-May-2025
Web References:
https://doi.org/10.1002/mog2.70022
Image Credits: The corresponding authors Dr. Xiang Chen, Dr. Jiachen Liu, and Dr. Yantao Xu.
Keywords: Malignant Melanoma, Adenosine Phosphate Signaling, Tumor Microenvironment, P2 Receptors, Immunotherapy, Immune Checkpoint Inhibitors, Multi-omics, Single-cell RNA Sequencing, Spatial Transcriptomics, Myeloid Cells, Antigen Presentation, Prognostic Biomarker