Converting CO2 into fuel and chemicals using electricity, also known as electrochemical conversion of CO2, is a promising way to reduce emissions. This process allows us to use carbon captured from industries and the atmosphere and turn it into resources that we usually get from fossil fuels.

Credit: Takuya Goto from Doshisha University
Converting CO2 into fuel and chemicals using electricity, also known as electrochemical conversion of CO2, is a promising way to reduce emissions. This process allows us to use carbon captured from industries and the atmosphere and turn it into resources that we usually get from fossil fuels.
To advance ongoing research on efficient electrochemical conversion, scientists from Doshisha University have introduced a cost-effective method to produce valuable hydrocarbons from CO2. The study was made available online on 17 May 2024 and formally published in the journal Electrochimica Acta on 20 July 2024. The research team, led by Professor Takuya Goto and including Ms. Saya Nozaki from the Graduate School of Science and Engineering and Dr. Yuta Suzuki from the Harris Science Research Institute, produced ethylene and propane on a basic silver (Ag) electrode by utilizing an ionic liquid containing metal hydroxides as the electrolyte.
“Most studies on CO2 electrolysis with room-temperature liquid electrolyte have focused on the electrode’s catalytic properties. In this groundbreaking study, we focused on the electrolyte and succeeded in producing valuable hydrocarbon gas even on a simple metal electrode,” says Prof. Goto.
Ionic liquids offer unique advantages for the electrochemical reduction of CO2. They operate over a wide range of voltages without decomposing, are non-flammable, and have high boiling points. This stability enables the electrolyte to withstand the high temperatures generated during exothermic CO2 reduction.
In their study, researchers investigated the electrochemical conversion of CO2 and water with N, N-diethyl-N-methyl-N-(2-methoxyethyl) ammonium tetrafluoroborate (DEME-BF4) as the electrolyte. The DEME-BF4 electrolyte provides optimal conditions for maximizing CO2 reduction. DEME+ ions enhance the solubility of CO2, allowing a greater number of CO2 molecules to participate in the reaction. Moreover, due to its hydrophilic nature, the hydrogen ions required for reducing CO2 to hydrocarbons can be easily supplied by mixing the electrolyte with water.
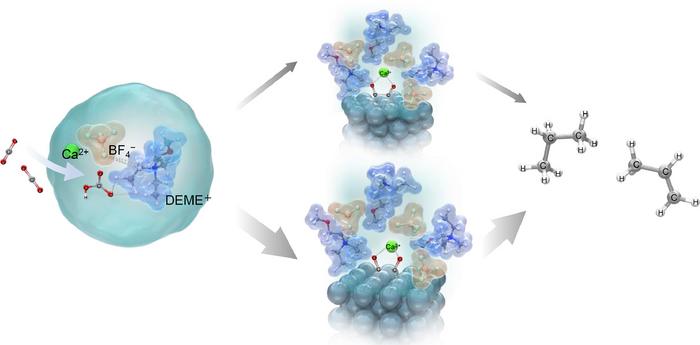
The researchers determined that the electrochemical conversion of CO2 to hydrocarbons could be increased with the addition of aqueous solutions containing metal hydroxides like calcium hydroxide (Ca(OH)2), sodium hydroxide (NaOH), and cesium hydroxide (CsOH) to the electrolyte. The hydroxides in the ionic liquid can react with CO2 to form bicarbonates (HCO3−) and carbonates (CO32−), further enhancing the availability of CO2 to participate in electrochemical reactions.
Under room temperature electrolysis (298 K or 25°C) in a CO2 atmosphere, the researchers successfully reduced CO2 to ethylene (C2H4), ethane (C2H6), propylene (C3H6), and propane (C3H8). They achieved the highest current efficiencies for each product using DEME-BF4 electrolyte mixed with water and containing Ca(OH)2, with efficiencies reaching up to 11.3% for propane and 6.49% for ethylene. This efficiency surpassed those obtained with other metal hydroxides by over 1000 times.
The reason for this high efficiency was explained using Raman spectroscopy and density functional theory (DFT) calculations. These analyses revealed that bicarbonate ions, formed when CO2 interacts with OH– ions in the electrolyte, interact with DEME+ and BF4– ions of the electrolyte to form a stable structure [DEME+-BF4−-HCO3−-Ca2+].
CO2 and HCO3– species then adsorb onto the electrode surface forming adsorbed species CO− ads. The adsorbed CO– ions then strongly interact with Ca2+ ions present in the electrolyte, forming two distinct intermediate structures: One structure A, consisting of a Ca2+ ion coordinated with two CO− ions adsorbed on three Ag atoms, and the other Structure B, where the Ca2+ ion is coordinated with two CO− ions adsorbed on two Ag atoms. This interaction with Ca2+ ions is crucial as it increases the stability of the adsorbed species, making the subsequent electrochemical reactions possible.
Among these structures, researchers suggest that structure B is more stable and is the preferred pathway for ethylene, while structure A leads to the production of propane. “We showed that tailoring the electrolyte can lead to molecular-level changes in the phase transformation of CO2 in bulk solution and at the electrode/ionic liquid electrolyte interface and proposed a process that enables the synthesis of unique hydrocarbons such as C3,” says Prof. Goto.
These findings shed light on the processes involved in the conversion of CO2 at the interface between ionic liquid-based electrolytes and metal electrodes, such as the role of calcium ions. Such insights can help in the development of electrolytes for the efficient production of useful hydrocarbons from CO2. “The physicochemical knowledge of this new route from CO2 decomposition to synthesizing useful hydrocarbons, as revealed in this study, will be instrumental in advancing CO2 utilization technology and contributing to academic progress in materials science.” concludes Prof. Goto.
About Professor Takuya Goto from Doshisha University, Japan
Takuya Goto is a Professor in the Faculty of Science and Engineering, Department of Environmental Systems Science. He specializes in research areas such as Energy/Earth resource engineering, energy science, and electrochemistry. Prof. Goto has published more than 94 papers in scientific journals, on topics that include molten salt electrolysis and the utilization of captured CO2. He received his Doctor of Energy Science degree from Kyoto University. For more information, visit his researcher profile at
Funding information
This research was partially supported by JSPS KAKENHI Grant Number JP22K14700 and the steel carbon neutrality research grant from The Iron and Steel Institute of Japan.
Media contact:
Organization for Research Initiatives & Development
Doshisha University
Kyotanabe, Kyoto 610-0394, JAPAN
E-mail:jt-ura@mail.doshisha.ac.jp
Journal
Electrochimica Acta
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Electrochemical synthesis of C2 and C3 hydrocarbons from CO2 on an Ag electrode in DEME-BF4 containing H2O and metal hydroxides
Article Publication Date
17-May-2024
COI Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.