A groundbreaking study published in the journal Aging-US has unveiled critical insights into the molecular mechanisms disrupting fat cell formation in Hutchinson-Gilford progeria syndrome (HGPS), a devastating premature aging disease. The investigation, led by Felix Quirin Fenzl and Karima Djabali of the Technical University of Munich, rigorously explores the role of microRNAs—specifically miR-145-5p and miR-27b-3p—in hindering adipogenesis, the process by which stem cells differentiate into adipocytes. This pioneering work illuminates molecular pathways contributing to the fat loss characteristic of HGPS, while laying groundwork for novel therapeutic approaches aimed at mitigating one of the syndrome’s most debilitating symptoms.
HGPS is a rare genetic disorder caused by mutations in the LMNA gene, resulting in the production of progerin, an abnormal lamin A protein that compromises nuclear structural integrity. Beyond the well-documented cardiovascular deterioration and musculoskeletal impairments, HGPS patients experience marked lipodystrophy—the pathological loss of subcutaneous fat—leading to severe metabolic complications. The mechanisms connecting progerin accumulation to defective adipogenesis, however, have remained obscure, hindering therapeutic development. The current research addresses this gap by profiling miRNA expression patterns associated with adipocyte differentiation deficits in HGPS.
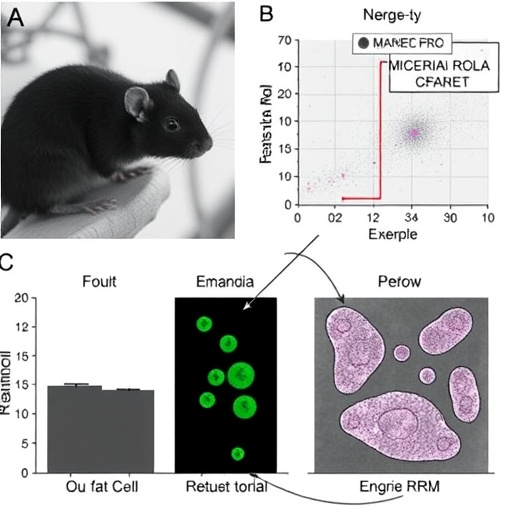
Employing fibroblast-derived induced pluripotent stem cells (iPSCs) from both HGPS patients and healthy controls, the researchers differentiated these stem cells into adipocytes and carefully analyzed miRNA activity at multiple stages of cell maturation. The results showcased a striking overexpression of miR-145-5p and miR-27b-3p in HGPS cells, correlating strongly with impaired adipogenic differentiation. These microRNAs were found to post-transcriptionally suppress key adipogenic transcription factors and markers, disrupting the tightly orchestrated gene regulatory networks required for the development of functional fat cells.
Further experiments confirmed that antagonizing miR-145-5p and miR-27b-3p via targeted inhibitors led to significant restoration of adipogenesis in HGPS-derived cells. This reversal was evidenced by increased expression of adipogenic markers such as PPARγ and FABP4, alongside improved lipid droplet accumulation. These findings not only establish a causative role for these microRNAs in the adipocyte maturation block but also spotlight them as promising molecular targets for therapeutic intervention aimed at mitigating lipodystrophy in premature aging.
The study also extended its findings in vivo, utilizing mouse models engineered to express progerin. Similar to human HGPS cells, adipose tissue from these mice exhibited elevated levels of miR-145-5p and miR-27b-3p concomitant with impaired fat deposition and metabolic dysfunction. This cross-species validation enhances the translational significance of the work and sets a robust foundation for future drug development targeting microRNA pathways to restore healthy adipose tissue architecture in HGPS.
By elucidating the interplay between aberrant microRNA expression and disrupted fat cell formation, the researchers shed light on a critical aspect of HGPS pathology that has eluded comprehensive understanding. The study broadens the therapeutic landscape beyond traditional approaches focused on managing cardiovascular and skeletal effects, redirecting efforts toward rectifying adipose tissue deficits that significantly impact patients’ quality of life and metabolic health.
Moreover, the implications of this research transcend HGPS, informing broader biomedical contexts involving adipose tissue dysfunction such as obesity, type 2 diabetes, and other metabolic syndromes. The identification of miR-145-5p and miR-27b-3p as pivotal regulators of adipogenesis may catalyze the development of microRNA-based therapeutics applicable to conditions where adipocyte differentiation and function are perturbed, highlighting the wider relevance of this investigation.
This comprehensive miRNA profiling study leverages state-of-the-art molecular biology techniques, including next-generation sequencing and functional inhibition assays, to map the regulatory networks that underlie adipogenic failure in HGPS. Such technical rigor strengthens the impact of the findings and promises to inspire subsequent research designed to translate these molecular insights into clinical reality.
Importantly, the research team emphasizes that while targeting miR-145-5p and miR-27b-3p offers a tantalizing therapeutic angle, challenges remain in delivering microRNA inhibitors effectively in vivo, ensuring tissue specificity, and circumventing off-target effects. Nonetheless, these hurdles are increasingly surmountable with advances in nanoparticle delivery systems and gene therapy vectors, providing optimism for clinical application in the near future.
In summary, the work by Fenzl and colleagues represents a significant advance in understanding the molecular etiology of lipodystrophy in Hutchinson-Gilford progeria syndrome. By delineating how deregulated microRNAs disrupt adipogenic pathways, this study not only enhances biological understanding of premature aging disorders but also propels the field toward novel treatments that could alleviate fat tissue loss and improve patient outcomes.
The discovery that miR-145-5p and miR-27b-3p serve as key impediments to adipogenesis creates an essential framework for developing microRNA-targeted therapies. Such strategies might one day restore adipose tissue function, counter metabolic deficits, and extend healthspan in children afflicted with HGPS and potentially other metabolic diseases.
Subject of Research: Cells
Article Title: Deregulated miR-145 and miR-27b in Hutchinson-Gilford progeria syndrome: implications for adipogenesis
News Publication Date: 27-Aug-2025
Web References: Aging-US Volume 17, Issue 9
References: The study integrates findings from multiple referenced works, specifically literature sources numbered 73 to 80 in the original paper.
Image Credits: Copyright © 2025 Fenzl et al. Distributed under Creative Commons Attribution License (CC BY 4.0)
Keywords: aging, Hutchinson-Gilford progeria syndrome (HGPS), progerin, microRNAs, adipogenesis