In a groundbreaking new study poised to reshape our understanding of Alzheimer’s disease, scientists have unveiled a pivotal role for a distinct subtype of immune cells known as CD103– CD8+ T cells in driving neurotoxic inflammation. Published in Nature Communications, this research elucidates how these cells contribute to neurodegeneration through a previously unrecognized signaling axis involving granzyme K and the protease-activated receptor-1 (PAR-1). This discovery not only offers profound insights into the inflammatory processes underpinning Alzheimer’s but also paves the way for novel therapeutic strategies targeting immune mechanisms in neurodegenerative disorders.
Alzheimer’s disease, a progressive neurodegenerative disorder characterized by memory loss and cognitive decline, has long been associated with pathological hallmarks such as amyloid-beta plaques and tau tangles. While much attention has focused on these protein abnormalities, increasing evidence implicates the immune system — and neuroinflammation in particular — as a key contributor to disease progression. Yet, the specific immune players and molecular pathways orchestrating this damaging inflammation have remained elusive. This study addresses that gap by identifying a specific immune cell phenotype that exacerbates neurotoxicity in Alzheimer’s brains.
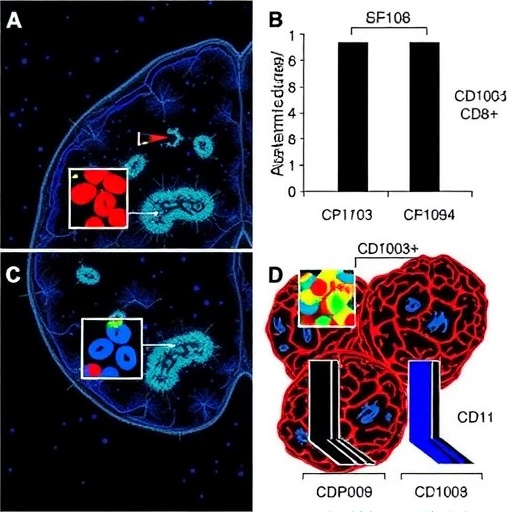
The research team centered their investigation on CD8+ T lymphocytes, critical components of the adaptive immune response traditionally known for their ability to kill infected or cancerous cells. Within this population, the researchers differentiated between cells expressing the integrin CD103 and those lacking it (CD103–). Using sophisticated flow cytometric analyses and brain tissue samples derived from Alzheimer’s patients, the scientists found an enrichment of CD103– CD8+ T cells infiltrating the central nervous system, particularly in regions burdened with neurodegenerative pathology.
Through rigorous molecular profiling, it became evident that these CD103– CD8+ T cells exhibit a unique pro-inflammatory signature distinct from their CD103+ counterparts. Most notably, they secrete elevated levels of granzyme K, a serine protease typically implicated in inducing apoptosis and inflammation. The production of granzyme K by these cells triggers downstream signaling pathways that potentiate neurotoxic inflammatory cascades, setting off a chain reaction detrimental to neuronal survival.
Central to the mechanism revealed is the activation of the protease-activated receptor-1 (PAR-1), a G-protein-coupled receptor widely expressed on neurons, microglia, and endothelial cells in the brain. The interaction between granzyme K and PAR-1 initiates signaling events that amplify neuroinflammation and compromise blood-brain barrier integrity, thereby exacerbating neuronal damage. This intricate cell-to-cell dialogue underscores a previously unappreciated axis through which adaptive immunity can influence neurodegeneration in Alzheimer’s disease.
Further experiments in murine models recapitulated key features of the human pathology, confirming that adoptive transfer of CD103– CD8+ T cells precipitates heightened neuroinflammation and cognitive deficits. Conversely, genetic ablation or pharmacological inhibition of granzyme K or PAR-1 significantly ameliorated these adverse effects, bolstering the therapeutic potential of targeting this axis. Such findings offer promising translational avenues for the development of interventions aimed at modulating immune cell-mediated neurotoxicity.
Beyond its immediate implications for Alzheimer’s pathology, this study challenges prevailing dogmas that primarily associate neuroinflammation with innate immune cells like microglia and astrocytes. By highlighting the influential role of adaptive immunity—specifically specialized T cell subsets—this work opens new conceptual frameworks for how chronic inflammation might be orchestrated in the aging brain and other neurodegenerative conditions.
The methodological rigor underpinning this research cannot be overstated. Employing high-dimensional cytometry, single-cell transcriptomics, and in vivo functional assays, the investigators provide a multidimensional view of immune cell phenotypes and their impact on neural circuitry. This integrative approach reveals the complex interplay between immune subsets and brain cellular components that underlies the neurodegenerative process.
An intriguing aspect of this study is the selective absence of CD103 expression on the identified pathogenic CD8+ T cells. CD103, an integrin known for mediating tissue residency of T cells, appears to delineate a functional dichotomy within brain-infiltrating CD8+ populations. The CD103– subset emerges as more pro-inflammatory and neurotoxic, suggesting that adhesion molecule expression profiles might be key determinants of immune cell behavior in neurological contexts.
Moreover, the granzyme K–PAR-1 signaling axis delineated here adds to the growing recognition of non-classical roles for cytotoxic proteases beyond cell death induction. Granzyme K, traditionally overshadowed by granzyme B in immune cytotoxicity, gains newfound prominence as a mediator of inflammatory signaling, expanding the repertoire of molecular effectors implicated in neurodegeneration.
The revelation that CD103– CD8+ T cells can penetrate the central nervous system and engage in deleterious interactions with resident neural cells underscores the permeability and immune accessibility of the brain in Alzheimer’s disease. These findings raise fundamental questions about how blood-brain barrier alterations and peripheral immune activation converge to foster an environment permissive to neurotoxic T cell infiltration.
Importantly, the identification of this immune axis offers practical implications for patient stratification and biomarker discovery. Quantifying levels of CD103– CD8+ T cells or granzyme K activity in cerebrospinal fluid could provide valuable indicators of inflammatory status and disease progression, enabling more precise therapeutic targeting.
Looking forward, this paradigm-shifting work calls for further exploration into how these pathogenic T cells are activated and recruited to the brain, what antigen specificities they possess, and how their function might be modulated in vivo. The development of specific inhibitors of granzyme K or PAR-1 signaling tailored for central nervous system delivery emerges as a compelling strategy to dampen harmful inflammation without broadly suppressing immune competence.
The wider scientific community has lauded this research for its innovative integration of immunology and neuroscience, highlighting it as a template for dissecting complex mechanisms of neurodegenerative disease. Furthermore, it exemplifies the power of interdisciplinary collaboration, combining expertise in immunology, molecular biology, neuropathology, and translational medicine.
In summary, the discovery of CD103– CD8+ T cells as critical mediators of neurotoxic inflammation via granzyme K and PAR-1 elucidates a novel immune-neural interface driving Alzheimer’s disease progression. This insight heralds a new frontier in therapeutic development, inspiring renewed optimism that modulating selective immune pathways can mitigate the burden of Alzheimer’s and transform patient outcomes in neurodegenerative disorders.
Subject of Research: Neuroinflammation and immune mechanisms in Alzheimer’s disease, focusing on CD103– CD8+ T cells and granzyme K–PAR-1 signaling.
Article Title: CD103– CD8+ T cells promote neurotoxic inflammation in Alzheimer’s disease via granzyme K–PAR-1 signaling.
Article References:
Terrabuio, E., Pietronigro, E.C., Bani, A. et al. CD103– CD8+ T cells promote neurotoxic inflammation in Alzheimer’s disease via granzyme K–PAR-1 signaling. Nat Commun 16, 8372 (2025). https://doi.org/10.1038/s41467-025-62405-6
Image Credits: AI Generated