In a groundbreaking study recently published in the Journal of Medical and Biological Engineering, researchers have illuminated a pivotal aspect of stem cell biology: the influence of mechanical forces on cellular behavior. In particular, the research focuses on the effects of cyclic stretch on the chondrogenic differentiation of rat adipose-derived stem cells (ADSCs). This compelling investigation highlights the role of biomechanical stimuli in tissue engineering and regenerative medicine, where understanding cell differentiation processes is crucial for developing effective therapies.
Adipose-derived stem cells, known for their ease of isolation and prolific capacity for differentiation, offer a promising resource for regenerative medicine. By harnessing these cells, scientists can create replacements for damaged or degenerated tissues, particularly in the context of cartilage repair. The research team, led by Lee et al., meticulously examined how applying mechanical cyclic stretch influences ADSCs’ journey toward becoming cartilage-like cells. This study promises significant implications for both basic cell biology and clinical applications.
Cyclic stretch is a biomechanical stimulus that mimics the natural mechanical environment experienced by cells within healthy tissues. Such stretch can be elicited from physical activities, with underlying mechanisms deeply ingrained in cellular signaling pathways. In the study, the researchers employed a PDMS (polydimethylsiloxane) membrane coated with Type-I collagen to provide a suitable environment for ADSC cultivation. The flexibility and biocompatibility of PDMS made it an ideal substrate to investigate the consequences of mechanical stretching.
Results showed that subjecting ADSCs to cyclic stretch significantly enhanced their chondrogenic differentiation. The researchers utilized a well-structured experimental design that involved various stretch parameters such as frequency and amplitude. Their findings reveal that specific conditions resulted in increased expression of chondrogenic markers, indicating that mechanical cues are integral to guiding stem cells’ fate. Notably, the study reinforces that understanding the interactions between mechanical stimuli and stem cells can lead to innovative strategies that enhance tissue regeneration.
Moreover, the authors discussed the underlying molecular mechanisms governing the cells’ responses to cyclic stretch. They highlighted the importance of mechanotransduction pathways, which convert mechanical signals into biochemical actions, ultimately influencing gene expression. The identification of key signaling molecules that mediate this process could pave the way for the development of targeted therapies aimed at improving cartilage repair strategies.
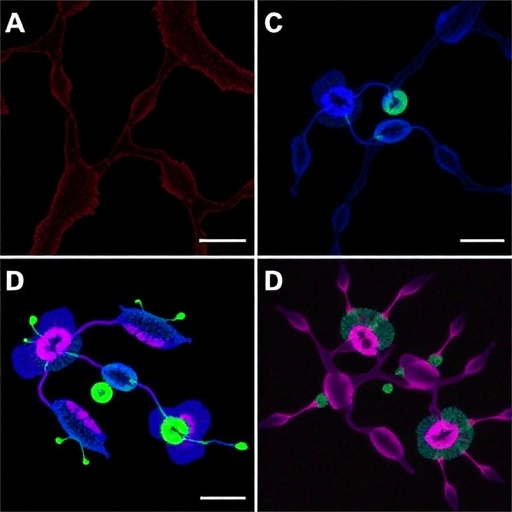
Further examination of cellular mechanics through advanced imaging techniques revealed insights into cell morphology changes under cyclic stretch conditions. The ADSCs exhibited notable alterations, becoming more elongated and clustered, resembling the natural architecture of cartilage tissue. These morphological adaptations suggest that mechanical forces not only impact gene expression but also enhance the physical characteristics necessary for cartilage function.
The research also delves into the role of the extracellular matrix (ECM) in chondrogenic differentiation. Type-I collagen, as a primary component of the ECM, plays a crucial role in providing structural support and biochemical cues to stem cells. By coating the PDMS membrane with this collagen type, the researchers created a more biologically relevant environment that likely influenced cell behavior, serving to bridge the gap between in vitro and in vivo conditions.
In terms of practical applications, this study offers invaluable insights for the optimization of tissue engineering strategies. As the clinical need for effective cartilage repair grows, strategies that incorporate biomechanical conditioning could lead to more effective therapies. By replicating the natural mechanical environments found in the body, researchers can potentially improve the engineering of cartilage tissues that are viable for transplantation.
Furthermore, the implications of these findings extend beyond cartilage regeneration. The principles of mechanobiology can be employed in various fields within regenerative medicine. By grasping how mechanical forces affect stem cell fate, researchers may be able to devise novel therapies for a multitude of conditions, including osteoarthritis, traumatic injuries, and other degenerative diseases.
In conclusion, the research conducted by Lee et al. marks a significant advancement in our understanding of stem cell biology, specifically regarding the chondrogenic differentiation driven by mechanical forces. The enhanced differentiation of ADSCs under cyclic stretch conditions not only elucidates the importance of mechanical signals in cellular behavior but also sets the groundwork for future research aimed at harnessing these principles for therapeutic applications.
This study, which emphasizes the interplay between biomechanics and stem cell biology, signifies a pivotal step toward integrating mechanical engineering principles into regenerative medicine practices. As the scientific community continues to explore the vast potential of stem cells, studies like this underscore the importance of interdisciplinary approaches that draw from both biology and engineering to address complex medical challenges.
The future of tissue engineering and regenerative medicine is bright, bolstered by discoveries such as those presented by Lee et al. As scientists deepen their understanding of the mechanical influences on stem cell differentiation, the hope is to engineer functional tissues that can withstand the stressors of daily life and ultimately improve patient outcomes in a wide range of clinical settings.
Subject of Research: Effects of Cyclic Stretch on Chondrogenic Differentiation of Rat Adipose-Derived Stem Cells
Article Title: Effects of Cyclic Stretch on Chondrogenic Differentiation of Rat Adipose-Derived Stem Cells Cultured on Type-I Collagen Coated PDMS Membrane
Article References: Lee, HM., Li, HY., Hsieh, YH. et al. Effects of Cyclic Stretch on Chondrogenic Differentiation of Rat Adipose-Derived Stem Cells Cultured on Type-I Collagen Coated PDMS Membrane. J. Med. Biol. Eng. (2025). https://doi.org/10.1007/s40846-025-00979-8
Image Credits: AI Generated
DOI: 10.1007/s40846-025-00979-8
Keywords: Chondrogenic differentiation, adipose-derived stem cells, cyclic stretch, tissue engineering, mechanotransduction.