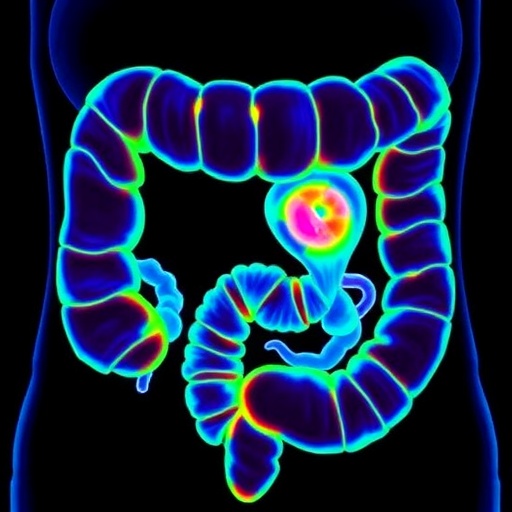
In a groundbreaking advancement that could revolutionize colorectal cancer diagnosis and treatment, researchers at the Champalimaud Foundation in Portugal have unveiled a novel, non-invasive imaging technique capable of accurately distinguishing cancerous from benign colorectal tissues in real time. Leveraging the natural autofluorescence emitted by biological tissues, combined with sophisticated machine learning algorithms, this innovative approach promises to enhance early cancer detection during colonoscopy or surgical procedures, potentially saving countless lives by enabling prompt, precise intervention.
Colorectal cancer (CRC) remains among the leading causes of cancer mortality worldwide, largely due to late-stage diagnosis and difficulties in distinguishing precancerous tissues during routine examination. Although traditional colonoscopy has greatly improved early lesion detection, current modalities struggle to ascertain which detected lesions carry malignancy risk without resorting to invasive biopsies, a process that can delay treatment and increase patient burden. Addressing this critical gap, the Champalimaud team harnessed autofluorescence lifetime imaging—a method that analyzes the temporal decay of endogenous fluorescent signals after targeted excitation by specific light wavelengths.
Autofluorescence, a property exhibited by various biomolecules such as collagen, NADH, and flavins, emits faint light without the need for exogenous dyes or contrast agents when stimulated with ultraviolet or visible light. By measuring the duration this fluorescence persists, known as fluorescence lifetime, researchers can infer subtle biochemical changes associated with malignant transformation. This label-free technique offers an intrinsic contrast mechanism, revealing molecular tissue architecture and metabolism with exceptional sensitivity.
The study employed a dual-laser excitation system emitting at 375 nm and 445 nm, designed to optimally excite a range of native fluorophores within the colorectal tissue microenvironment. Fresh samples from 117 patients undergoing colorectal resections were probed with a fiber-optic setup capable of capturing multiparametric autofluorescence lifetime data across several spectral channels. These rich datasets were meticulously correlated with gold standard histopathological analyses to create a comprehensive training library for computational modeling.
Crucially, the team applied an ensemble learning strategy, Adaptive Boosting (AdaBoost), to classify tissue types based on discriminative lifetime features extracted from the spectroscopic signatures. This approach aggregates multiple weak classifiers to forge a strong predictive model, adept at handling the complexity and heterogeneity inherent in biological tissues. On training data, the classifier achieved an impressive accuracy of 87%, complemented by a sensitivity of 83% and a specificity of 90%, indicating robust discrimination between malignant and non-malignant samples.
Validation on independent test sets demonstrated consistent performance, with the model attaining 85% accuracy, 85% sensitivity, and 85% specificity, underscoring its generalizability and potential clinical applicability. Remarkably, the system could generate detailed probability maps of tissue malignancy at the single measurement point scale, highlighting tumor regions with spatial precision that could be invaluable for surgical guidance or targeted biopsy planning.
Importantly, the researchers explored the feasibility of simplifying the optical instrumentation by reducing the number of monitored spectral channels. The findings revealed that focusing on the most biochemically informative autofluorescence lifetimes still sustained high classification efficacy. This simplification could pave the way for cost-effective, compact clinical devices adaptable for widespread use, overcoming current logistical and financial barriers.
Although the results are highly promising, the authors acknowledge that further refinement is necessary, particularly in enhancing sensitivity for early-stage or borderline neoplastic lesions, which often present subtle biochemical distinctions. Moreover, broader clinical validation across diverse patient demographics will be pivotal in demonstrating the robustness of this modality in routine practice.
Beyond cancer detection, this study exemplifies the growing convergence of optical biophotonics and artificial intelligence, illustrating how quantitative, label-free optical signatures can serve as biomarkers for disease states. The integration of machine learning enables real-time, automated interpretation of complex datasets, facilitating rapid clinical decision-making without additional procedural burden.
The potential impact of this technology is expansive. By reducing reliance on invasive biopsies, promoting targeted interventions, and shortening procedural times, it promises to enhance patient comfort and healthcare efficiency. Early and accurate identification of malignant tissue during colonoscopy could improve cure rates and mitigate the significant morbidity associated with advanced colorectal cancers.
As this innovative approach evolves, it opens pathways for extending autofluorescence lifetime imaging combined with AI to other gastrointestinal malignancies and perhaps broader oncological applications. The harmonization of optical engineering, molecular pathology, and computational analytics embodied by this work exemplifies a new frontier in precision medicine.
This pioneering research not only underscores the utility of endogenous optical signals as rich diagnostic assets but also embodies a paradigm shift towards minimally invasive, data-driven oncology. Its translation from bench to bedside could mark a significant milestone in colorectal cancer management, aligning with global health priorities to reduce cancer burden through technological innovation.
For those invested in the future of medical imaging and cancer diagnostics, the Champalimaud Foundation’s study represents an inspiring blueprint for harnessing the subtle interplay of light and tissue biochemistry, amplified by machine learning intelligence, to deliver transformative clinical tools.
Subject of Research: Human tissue samples
Article Title: Identification of colorectal malignancies enabled by phasor-based autofluorescence lifetime macroimaging and ensemble learning
News Publication Date: 4-Jul-2025
References:
Lagarto J. L. et al., “Identification of colorectal malignancies enabled by phasor-based autofluorescence lifetime macroimaging and ensemble learning,” Biophotonics Discovery, vol. 2, no. 3, 032705, 2025. DOI: 10.1117/1.BIOS.2.3.032705
Image Credits:
Image courtesy of J. Lagarto (Champalimaud Foundation).
Keywords:
Cancer research, Biotechnology, Medical imaging, Data analysis