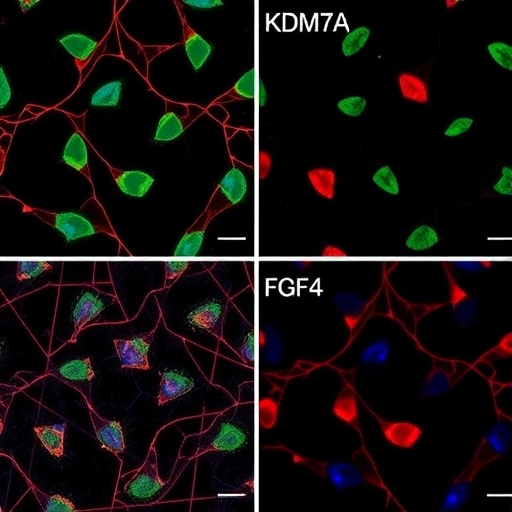
In the rapidly evolving field of neurobiology, the intricate processes governing neural differentiation have long been a subject of fascination and intensive research. Recently, a groundbreaking correction published in Cell Research has cast new light on the role of the dual-specificity histone demethylase KIAA1718, also known as KDM7A, in orchestrating neural differentiation via the fibroblast growth factor 4 (FGF4) pathway. This revelation not only refines our understanding of epigenetic regulation in neural development but also opens promising avenues for therapeutic intervention in neurodevelopmental disorders. The corrected insights provided by Huang, Xiang, Wang, and their colleagues illuminate critical molecular crosstalk that could redefine therapeutic strategies targeting cellular plasticity and neurogenesis.
Epigenetics—the study of heritable changes in gene function without alterations in DNA sequence—has emerged as a pivotal domain for decoding cellular differentiation. Among epigenetic mechanisms, histone modifications are paramount, influencing chromatin architecture and gene expression patterns. KDM7A, a histone demethylase with dual specificity, targets methyl groups on histones H3K9me2 and H3K27me2. These specific lysine residues are key repressive marks that regulate transcriptional silencing. By selectively removing these repressive methylations, KDM7A activates gene expression programs essential for lineage specification. The refined understanding of KDM7A’s demethylase activity, as elucidated in the recent correction, emphasizes its central role in neural lineage commitment, heralding a more complex epigenetic regulatory network than previously appreciated.
FGF4, a member of the fibroblast growth factor family, has long been recognized for its critical functions in embryonic development, cell proliferation, and differentiation. Its involvement in neural differentiation is especially significant, given that FGF4 signaling orchestrates a cascade of downstream pathways promoting neurogenesis and cellular maturation. The synergy between epigenetic modulators like KDM7A and morphogenetic factors such as FGF4 forms a sophisticated regulatory axis that finely tunes neural progenitor fate decisions. The latest findings by Huang et al. detail this functional interaction, demonstrating that KDM7A positively regulates FGF4 expression through targeted demethylation, thereby fostering a conducive chromatin environment for neural differentiation gene programs.
Diving deeper into the mechanistic aspects, KDM7A’s dual demethylase activity is uniquely suited to regulate repressive histone marks that stabilize a closed chromatin conformation. By orchestrating the removal of dimethyl groups from both H3K9 and H3K27 residues, KDM7A facilitates chromatin remodeling necessary for transcriptional activation of neural-specific genes. This dual specificity differentiates it from monofunctional demethylases and highlights a coordinated epigenetic mechanism that ensures precise temporal and spatial gene expression during neurodevelopment. The correction clarifies that this concerted demethylase action directly influences FGF4 promoter accessibility, a key insight that revises prior models of KDM7A function.
Moreover, the regulatory relationship between KDM7A and FGF4 exemplifies the intricate balance between gene repression and activation that underpins cellular differentiation. During early neural differentiation, progenitor cells experience epigenetic remodeling, where the repression marks H3K9me2 and H3K27me2 are dynamically regulated. KDM7A’s involvement in this epigenetic switch ensures that the critical threshold of FGF4 expression is met to drive neural fate commitment effectively. Importantly, aberrant regulation of such pathways has been implicated in various neurodevelopmental anomalies, underscoring the clinical relevance of this regulatory axis.
The implications of the corrected findings extend into the realm of stem cell biology and regenerative medicine. Understanding how KDM7A modulates FGF4 offers potential strategies to manipulate neural progenitor cells for therapeutic purposes. By modulating KDM7A activity, researchers could enhance the generation of functional neural cells from pluripotent stem cells, paving the way for innovative treatments of neurodegenerative diseases and injury repair. Furthermore, the specificity of KDM7A’s histone demethylase activity provides a potential target for small molecules that could refine neural differentiation outcomes with precision.
Expanding on the broader context, the modulation of histone methylation states by enzymes like KDM7A contributes to the plasticity of the epigenome during development. The dual specificity demethylation mechanism ensures a rapid and robust response to developmental cues, allowing cells to transition efficiently from a pluripotent to a differentiated state. This finely tuned epigenetic regulation is critical not only in normal development but also in pathological states where differentiation is dysregulated, such as in cancer or neurodegenerative diseases.
Technological advances, including chromatin immunoprecipitation followed by sequencing (ChIP-seq), have allowed the precise mapping of KDM7A binding sites genome-wide, revealing its preferential localization to promoters and enhancers of genes involved in neurogenesis. Integrating these epigenomic data with transcriptomic profiles further clarifies how KDM7A-mediated demethylation at the FGF4 locus upregulates expression during neural commitment. The correction issued refines the understanding of these data sets, ensuring the scientific narrative accurately reflects the enzyme’s functional landscape.
The interplay between epigenetic modifiers such as KDM7A and signaling factors like FGF4 also serves as a paradigm illustrating how gene-environment interactions shape neural development. Environmental factors influencing epigenetic modifications may impact KDM7A activity or expression, thereby modulating FGF4 signaling and ultimately affecting neurodevelopmental trajectories. Such insights open discussions about how external stimuli, ranging from nutrition to toxins, may intersect with the epigenetic machinery to influence brain development and function.
Notably, the correction addressed by Huang and colleagues underscores the importance of scientific rigor and transparency in the rapidly progressing field of epigenetics. It highlights that as we delve deeper into the multi-layered regulatory networks governing cellular differentiation, continuous validation and refinement of data are paramount. This dedication to accuracy ensures that subsequent research, drug development, and clinical applications are built on a solid foundation of reproducibility and clarity.
Looking forward, unraveling the full spectrum of KDM7A’s substrates and interacting partners remains a critical avenue for research. While the current correction focuses on its role in neural differentiation via FGF4, it is plausible that KDM7A modulates additional pathways imperative for neuronal maturation, synaptic plasticity, or even glial cell functions. Elucidating these would significantly broaden the therapeutic potential of targeting KDM7A in neurological diseases.
In addition, the dual-specificity nature of KDM7A invites deeper biochemical investigations into its structural domains and catalytic mechanisms. Understanding how this enzyme discriminates between different histone marks and how its activity is regulated post-translationally could inform the design of selective inhibitors or activators. Such molecules would be invaluable for dissecting epigenetic regulation in vitro and in vivo, as well as for clinical modulation of neural differentiation processes.
Complementary to molecular insights, integrating KDM7A-FGF4 axis studies into developmental models, such as organoids or transgenic animals, will reveal physiological consequences and context-dependent effects. Such models can simulate complex tissue environments and developmental timelines, providing holistic perspectives on how epigenetic and signaling networks converge to sculpt the nervous system.
The corrected work also resonates with parallel research exploring epigenetic regulation in other organ systems and disease paradigms. Given that fibroblast growth factors and histone demethylases are broadly involved in tissue patterning and homeostasis, the principles elucidated through studying KDM7A and FGF4 in neural differentiation may have far-reaching implications. This includes cancer biology, where epigenetic dysregulation and altered growth factor signaling often coexist.
In conclusion, the author correction presented in Cell Research by Huang et al. enriches our understanding of the epigenetic regulation of neural differentiation. By clarifying the role of KDM7A as a dual-specificity histone demethylase that modulates FGF4 expression, the study highlights a sophisticated molecular mechanism fundamental to neurodevelopment. This nuanced insight not only advances basic science but also provides a compelling framework for therapeutic innovations in regenerative medicine and neurodevelopmental disorders. As we continue to decipher the epigenome’s language, findings like these emphasize the delicate interplay between chromatin dynamics and cellular fate decisions, a narrative that will undoubtedly catalyze future breakthroughs in neuroscience.
Subject of Research: Neural differentiation regulated by epigenetic enzyme KDM7A via FGF4 signaling.
Article Title: Author Correction: Dual-specificity histone demethylase KIAA1718 (KDM7A) regulates neural differentiation through FGF4.
Article References:
Huang, C., Xiang, Y., Wang, Y. et al. Author Correction: Dual-specificity histone demethylase KIAA1718 (KDM7A) regulates neural differentiation through FGF4.
Cell Res (2025). https://doi.org/10.1038/s41422-025-01143-2
Image Credits: AI Generated