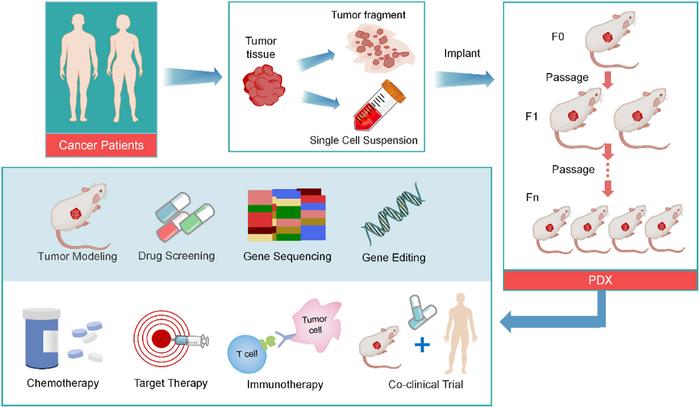
Cancer remains a formidable adversary in global health, affecting millions annually and presenting persistent challenges to effective treatment. Despite significant advances through precision medicine and targeted therapies that have reshaped oncology, the issues of drug resistance and disease recurrence continue to plague many patients. A seminal review recently published in Genes & Diseases sheds light on the revolutionary potential of patient-derived xenograft (PDX) models as a transformative preclinical platform that more accurately replicates the complexity of human tumors. This advancement holds the promise to dramatically alter drug development pipelines and personalized cancer therapy paradigms.
PDX models originate by engrafting freshly resected human tumor specimens directly into immunodeficient murine hosts. This method preserves the heterogeneity of tumor genetics, the intricate tumor microenvironment, and dynamic drug responsiveness that traditional cell line models fail to capture. Cell lines often lose critical tumor-specific features through prolonged in vitro culture, but PDXs maintain the malignant phenotype in vivo, providing a more faithful and clinically relevant experimental framework. Consequently, PDX models have emerged as an indispensable asset for investigating novel therapeutic strategies prior to clinical trials.
Crucially, PDX models permit the conduct of co-clinical trials, a revolutionary approach where patients receive treatment concurrently with their personalized PDX avatars. This parallel testing enables real-time assessment of therapeutic efficacy, facilitating rapid adaptation of clinical interventions tailored for individual patients. By integrating clinical decision-making with rigorous preclinical validation, this strategy advances the precision medicine vision from concept to clinical application. Notably, PDX models have already yielded critical insights in breast, lung, colorectal, and ovarian cancers, among other malignancies.
Despite their promise, PDX models confront substantial hurdles that have limited their widespread adoption. The complexity of establishing such models demands high costs and extended engraftment periods, requiring access to specialized animal facilities and skilled personnel. Additionally, genetic and epigenetic drift can occur within the murine host, potentially diverging from the evolution observed in patient tumors over time. This discrepancy poses challenges for modeling long-term disease progression and resistance mechanisms, necessitating ongoing efforts to refine system fidelity.
To transcend current limitations, innovative next-generation PDX platforms are under development. Integrating cutting-edge technologies like CRISPR-Cas9 gene editing allows for precise manipulation of tumor genomes within PDXs, enabling in-depth functional studies of oncogenic drivers and resistance pathways. Coupling PDX models with organoid co-cultures offers a hybrid system to examine tumor-stroma interactions and drug responses ex vivo while maintaining physiological relevance. Furthermore, humanized mouse models, equipped with reconstituted human immune systems, provide powerful tools for evaluating immunotherapy responses within the PDX framework.
Biobanking of patient-derived tumors coupled with artificial intelligence-driven analytics is accelerating PDX model utility. High-throughput sequencing and machine learning algorithms facilitate comprehensive characterization of PDX molecular profiles, predicting therapeutic vulnerabilities with unprecedented accuracy. These advances not only expedite drug discovery and validation but also enable stratified medicine approaches that select optimal therapies based on tumor-specific signatures captured by PDX models. Such integration is poised to reshape oncological drug development paradigms fundamentally.
Another advantage of PDX systems lies in their ability to test combination therapies and adaptive dosing regimens in a highly personalized context. By recapitulating patient-specific tumor biology, PDXs allow researchers to dissect mechanistic pathways driving therapeutic synergy or resistance. This capability is invaluable for developing next-generation regimens that circumvent resistance mechanisms and enhance durable responses. The fine-tuned modeling of interpatient variability enhances the translational relevance of PDX-derived data, informing clinical trial design more effectively.
However, ethical considerations and logistical constraints still pose barriers to PDX model scalability. The reliance on immunodeficient rodents warrants careful consideration of welfare and reduction strategies in animal research. Advances in three-dimensional culture systems and in silico modeling may eventually complement or, in part, replace PDX usage, but for now, PDXs remain unparalleled in their predictive power for human oncological applications. Continued investment in infrastructure and collaborative frameworks is essential to democratize access to these powerful models in the research community.
Furthermore, the heterogeneity of tumor microenvironments within PDXs underscores the importance of careful experimental design and interpretation. Infiltrating stromal cells and vasculature components derive from host murine tissue, which can influence tumor behavior and therapeutic responses differently from the native human microenvironment. Addressing this issue through humanization protocols or co-implantation strategies is a fertile area of ongoing research, aiming to recreate a more authentic tumor niche and improve translational validity.
In light of mounting evidence, the role of PDX models as a cornerstone of precision oncology is increasingly apparent. As cancer biology research confronts the multifaceted nature of malignancies, PDX systems offer unparalleled opportunities for dissecting tumor complexity and tailoring therapeutic interventions. Given their ability to bridge experimental findings with clinical realities, these models are set to become standard tools in oncological research, drug development pipelines, and personalized patient care algorithms worldwide.
The convergence of emerging genomic editing technologies, immune-oncology advancements, and computational biology ensures that PDX models will evolve rapidly to meet future challenges. By embracing these multifaceted innovations, researchers are positioning PDX platforms not only as experimental stand-ins but as predictive engines fueling next-generation cancer therapies. Through this lens, the dynamic landscape of cancer precision medicine will be sharpened significantly, ultimately improving patient outcomes and survival rates.
As the oncology community moves forward, continued collaboration between clinicians, basic researchers, and biotechnology developers will be critical in harnessing the full potential of PDX models. Investing in the optimization, standardization, and dissemination of these models globally will accelerate translational breakthroughs. Together, these coordinated efforts herald a new era where cancer treatment becomes increasingly personalized, efficient, and successful—a testament to the power of patient-derived xenograft models in revolutionizing cancer therapeutics.
Subject of Research:
Patient-derived xenograft (PDX) models in cancer research and their role in precision oncology.
Article Title:
Patient-derived xenograft models: Current status, challenges, and innovations in cancer research
News Publication Date:
2025
References:
Minqi Liu, Xiaoping Yang, Patient-derived xenograft models: Current status, challenges, and innovations in cancer research, Genes & Diseases, Volume 12, Issue 5, 2025, 101520.
Image Credits:
Genes & Diseases
Keywords:
Cancer genetics, patient-derived xenograft models, precision medicine, drug resistance, tumor microenvironment, CRISPR gene editing, humanized mouse models, organoid co-cultures, co-clinical trials, biobanking, artificial intelligence in drug discovery, immuno-oncology.