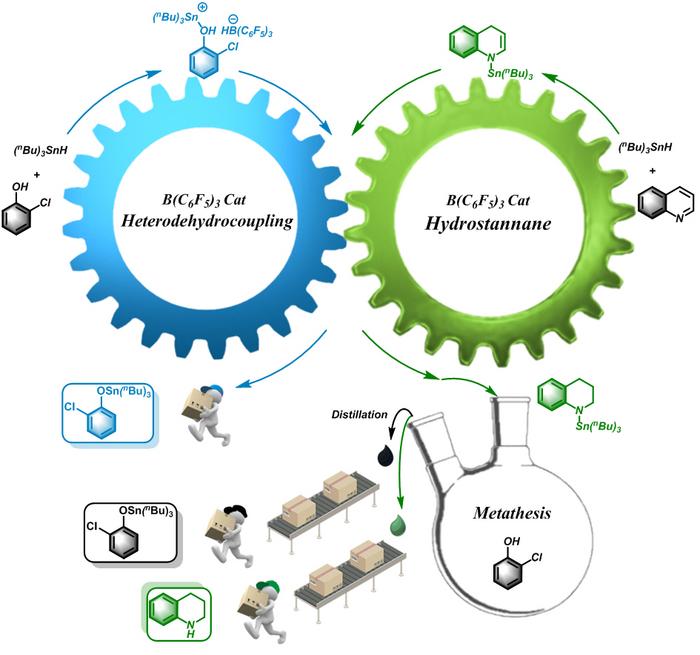
Heteroatom tin compounds (SSn, OSn, NSn, PSn) composed of heteroatoms S, O, N, P and tin atoms have attracted intense attention due to their wide applications in organic synthesis and pharmaceutical fields. The current methods for synthesis of such compounds, such as metathesis reactions, addition reactions, and free radical reactions, exhibit drawbacks including narrow substrate scope and harsh conditions. Therefore, it is important to develop efficient synthetic systems to construct heteroatom-tin bond.

Credit: Chinese Journal of Catalysis
Heteroatom tin compounds (SSn, OSn, NSn, PSn) composed of heteroatoms S, O, N, P and tin atoms have attracted intense attention due to their wide applications in organic synthesis and pharmaceutical fields. The current methods for synthesis of such compounds, such as metathesis reactions, addition reactions, and free radical reactions, exhibit drawbacks including narrow substrate scope and harsh conditions. Therefore, it is important to develop efficient synthetic systems to construct heteroatom-tin bond.
Tetrahydroquinoline, as an important organic synthetic intermediate and pharmaceutical intermediate, is of significant importance in the fields of biochemistry and pharmaceutical chemistry. The commonly used method is to reduce the quinoline to tetrahydroquinoline by H2, but often require harsh reaction conditions, such as high temperature and pressure. Therefore, it is highly demanded to develop a highly efficient reaction system to realize the reduction of quinoline to tetrahydroquinoline under mild conditions.
In nature, there is sometimes a mutually beneficial relationship between two or more species. For example, a sea anemone is attached to the shell of a host crab and carried by the host crab, allowing it to capture foods more effectively. Meanwhile, a sea anemone uses the toxic thorn cells to protect the host crab from attacks from natural enemies.
Here an organic synthetic strategy based on mutualism is reported by a research team led by Prof. Yuetao Zhang of Jilin University, China, in which the heterodehydrocoupling of hydrostanane and the reduction of quinolines promote each other, consuming high-energy intermediates and reducing reaction energy while expanding the substrate scope, furnishing a series of heteroatom-tin compounds and tetrahydroquinolines. The application of mutualism in organic synthesis enabled the simultaneous accomplishment of two reactions that used to be difficult or unlikely to occur, through the mutual promotion of each other. The successful application of this mutualism concept to the organic synthesis would definitely inspire more “impossible reactions” to be tackles in the future. The results were published in the Chinese Journal of Catalysis (
###
About the Journal
Chinese Journal of Catalysis is co-sponsored by Dalian Institute of Chemical Physics, Chinese Academy of Sciences and Chinese Chemical Society, and it is currently published by Elsevier group. This monthly journal publishes in English timely contributions of original and rigorously reviewed manuscripts covering all areas of catalysis. The journal publishes Reviews, Accounts, Communications, Articles, Highlights, Perspectives, and Viewpoints of highly scientific values that help understanding and defining of new concepts in both fundamental issues and practical applications of catalysis. Chinese Journal of Catalysis ranks among the top one journals in Applied Chemistry with a current SCI impact factor of 16.5. The Editors-in-Chief are Profs. Can Li and Tao Zhang.
Journal
Chinese Journal of Catalysis
Article Title
Mutualism in organic synthetic chemistry: Simultaneous heterodehydrocoupling of hydrostannane and reduction of quinoline
Article Publication Date
21-Mar-2024