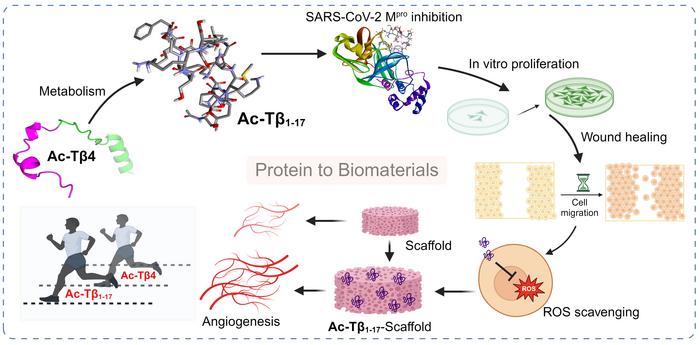
A groundbreaking advancement in antiviral therapeutics and regenerative medicine has emerged from the Korea Institute of Science and Technology (KIST), where researchers have developed a bioactive peptide derived from a native human protein with potent dual functionality. This peptide metabolite, named Ac-Tβ1-17, originates from thymosin β4, a naturally occurring protein recognized for its role in cellular processes. The research offers transformative potential by combining antiviral efficacy against the COVID-19 virus with the ability to stimulate tissue regeneration, marking a new frontier in multifunctional drug development.
Since the onset of the COVID-19 pandemic, the scientific community’s quest for effective antiviral agents has intensified dramatically. Meanwhile, the pharmaceutical industry has witnessed an elevated interest in peptide-based therapeutics due to their specificity, biocompatibility, and reduced side effects. Among these, peptides derived from endogenous proteins have captured attention as promising candidates, primarily because they leverage naturally occurring molecular frameworks, reducing immunogenic risks and improving therapeutic potential. KIST’s latest development capitalizes on these advantages, revealing how naturally fragmented peptides can be repurposed into powerful biomedical tools.
At the core of their study, the research team isolated Ac-Tβ1-17, a peptide metabolite resulting from the enzymatic breakdown of thymosin β4 within the human body. Thymosin β4 itself is well-known for its involvement in wound healing, angiogenesis, and inflammation modulation. However, understanding its metabolites’ distinct bioactivity had remained elusive until this pivotal investigation. By meticulously characterizing Ac-Tβ1-17, the scientists demonstrated that this fragment maintains and even enhances the parent protein’s regenerative functions while also acquiring substantial antiviral activity, particularly against SARS-CoV-2, the causative agent of COVID-19.
The team’s viral inhibition assays revealed that Ac-Tβ1-17 significantly suppresses the activity of the SARS-CoV-2 main protease, Mpro, a crucial enzyme required for viral replication. Remarkably, the peptide inhibited Mpro activity by more than 85%, indicating its robust potential as an antiviral agent. This discovery is particularly noteworthy, as therapeutic strategies targeting viral proteases have emerged as effective antiviral approaches. The bioactive peptide’s mechanism appears to involve direct binding interference with Mpro’s catalytic sites, thereby halting the replication cycle of the virus.
Beyond the antiviral capacity, Ac-Tβ1-17 demonstrated profound effects on cellular regeneration pathways. Experiments conducted on human vascular endothelial cells illuminated its capacity to accelerate cell proliferation, promote wound closure, stimulate angiogenesis, and reduce oxidative stress by scavenging reactive oxygen species (ROS). These regenerative properties align with the natural physiological roles of thymosin β4 while highlighting the peptide’s unique capability to amplify these effects within damaged or inflamed tissues, indicating its suitability for therapeutic interventions in tissue repair.
Translating these molecular and cellular findings into practical therapeutic applications required a versatile delivery platform. The researchers innovatively fabricated peptide-based scaffolds incorporating Ac-Tβ1-17, which serve as three-dimensional structural matrices supporting cell adhesion and growth. Such scaffolds are instrumental in regenerative medicine as they mimic the extracellular matrix, providing mechanical support and biochemical cues essential for tissue formation. Testing these scaffolds confirmed their efficacy in promoting vascular tissue regeneration, sustained cell viability, and enhanced blood vessel formation, thereby validating their clinical relevance.
What sets this study apart is the demonstration that a single, small peptide can concurrently execute both antiviral and pro-regenerative functions. This multifunctionality addresses a critical challenge in drug development, where therapeutics typically target one specific pathway. By integrating antiviral and tissue-restorative actions, Ac-Tβ1-17 offers a holistic approach to treating viral infections like COVID-19, which often cause extensive tissue damage alongside viral proliferation. Such dual-action agents could reduce treatment complexity and improve patient outcomes.
Moreover, the research underscores the untapped potential of endogenous protein metabolites as a novel drug source. Traditionally, drug discovery focuses on intact proteins or synthetic molecules, but this study illuminates how the human body’s own metabolic processes generate bioactive fragments with distinct and beneficial functions. Harnessing these metabolites could revolutionize biomaterials and therapeutic pipelines, bridging natural biochemistry with engineered medical solutions.
Future research directions outlined by the KIST team aim to optimize the therapeutic potential of Ac-Tβ1-17. Efforts will focus on tailoring peptide variants for enhanced stability, bioavailability, and targeted delivery. Additionally, exploring its applications beyond antiviral therapy, such as chronic wound healing, cardiovascular diseases, and inflammatory conditions, could widen its clinical impact. The development of personalized therapeutics utilizing this peptide scaffold also represents an exciting avenue to tailor treatments according to patient-specific needs.
The collaboration across multiple KIST research centers emphasized the interdisciplinary nature of this breakthrough, combining expertise in biomaterials, natural product biology, and molecular pharmacology. Such integrated approaches are crucial for advancing peptide therapeutics from bench to bedside. Furthermore, support from the Ministry of Science and ICT and the Global Research Network Program highlighted the strategic importance of this work within Korea’s scientific innovation framework.
Commenting on the significance of the findings, Dr. Hyung-Seop Han noted that the study validates the utility of protein metabolites not only as novel drugs but also as versatile biomaterials that can foster tissue regeneration. This dual functionality could profoundly influence future medical treatments designed to combat infections while simultaneously repairing affected tissues. Dr. Dae-Geun Song expressed optimism regarding the broader impacts of utilizing natural bioactive peptides in designing antivirals and regenerative biomaterials, promising new horizons in biomedical research. Meanwhile, Dr. Oh-Seung Kwon emphasized the potential for these discoveries to guide future drug development pipelines and expand therapeutic options for multifaceted diseases.
In conclusion, the discovery and development of Ac-Tβ1-17 mark a pivotal milestone in peptide therapeutics, representing a new class of bioactive molecules capable of addressing complex clinical challenges posed by viral pandemics and tissue damage. This naturally derived peptide not only challenges existing paradigms that separate antiviral and regenerative medicine but also sets the stage for multivalent therapeutic platforms that may transform the landscape of disease treatment and tissue engineering. As research progresses, Ac-Tβ1-17 may well become a cornerstone in the future toolkit against infectious diseases and tissue degeneration.
Subject of Research: Development and characterization of a bioactive peptide metabolite from thymosin β4 exhibiting antiviral and pro-regenerative activities.
Article Title: Protein to biomaterials: Unraveling the antiviral and proangiogenic activities of Ac-Tβ1-17 peptide, a thymosin β4 metabolite, and its implications in peptide-scaffold preparation
News Publication Date: 19-Mar-2025
Web References: DOI: 10.1016/j.bioactmat.2025.02.008
Image Credits: Korea Institute of Science and Technology
Keywords: Ac-Tβ1-17, thymosin β4 metabolite, antiviral peptide, SARS-CoV-2 Mpro inhibition, tissue regeneration, angiogenesis, wound healing, peptide scaffold, biomaterials, regenerative medicine, peptide therapeutics, bioactive peptides