Z-alkenes are organic compounds with a double bond between two carbon atoms and two substituents attached to the carbon atoms on the same side of the double bond. They are ubiquitous structural components of organic compounds in chemistry and biology. It is well known that many of the Z-alkenes cannot be prepared through conventional methods involving thermodynamic methods while photoisomerization can offer good yields. Photoisomerization is a process in which the structural arrangement of an isomer of a molecule is changed to another isomer by absorption of light. The photoisomerization of E-alkenes to produce Z-alkenes has many applications in the fields of organic chemistry, polymer chemistry, and medicinal chemistry.

Credit: Hideyo Takahashi from Tokyo University of Science
Z-alkenes are organic compounds with a double bond between two carbon atoms and two substituents attached to the carbon atoms on the same side of the double bond. They are ubiquitous structural components of organic compounds in chemistry and biology. It is well known that many of the Z-alkenes cannot be prepared through conventional methods involving thermodynamic methods while photoisomerization can offer good yields. Photoisomerization is a process in which the structural arrangement of an isomer of a molecule is changed to another isomer by absorption of light. The photoisomerization of E-alkenes to produce Z-alkenes has many applications in the fields of organic chemistry, polymer chemistry, and medicinal chemistry.
Many studies have explored different methods for photoisomerization of E-to-Z alkenes. A notable approach involved a continuous-flow system, in which the photosensitizer was immobilized in an ionic liquid and continuously recycled via a simple phase separation process. Photosensitizers are materials that enhance the rate of photoisomerization reactions by absorbing light energy and transferring it to the reactant molecule. However, current methods based on the use of ionic liquids are time-consuming and difficult to apply to recycling high-performance liquid chromatography (HPLC) technology, which enables the recycling of samples and therefore can enhance the efficiency of photoisomerization.
Inspired by these findings, in a new study, a team of researchers from Japan, led by Professor Hideyo Takahashi from the Faculty of Pharmaceutical Sciences at Tokyo University of Science, explored the photoisomerization of E-cinnamamides to Z-cinnamamides using a recycling photoreactor coupled with an HPLC system. The study, published online in The Journal of Organic Chemistry on June 05, 2024, included contributions from Ms. Mayuko Suga, Ms. Saki Fukushima, and Assistant Professor Dr. Kayo Nakamura, also from Tokyo University of Science.
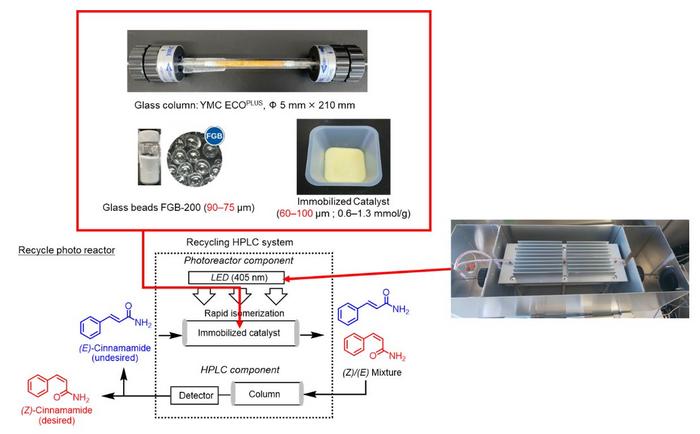
The team had previously developed a recycling photoreactor based on the deracemization concept. Prof. Takahashi explains, “Our closed-loop recycling photoreactor was initially used to convert a racemate, a mixture of left and right-handed enantiomers of a chiral molecule, into the pure desired enantiomer. It consists of a photocatalyst immobilized on a resin, which converts an undesired enantiomer into a racemate, and an HPLC column which separates the desired enantiomer. In this study, we adapted this method to convert E-cinnamamides to their Z-isomers.”
To employ this method for E-to-Z photoisomerization of alkenes, a photosensitizer that promotes rapid photoisomerization is required. To this end, the researchers screened several commercially available photosensitizers and identified thioxanthone as the best candidate. Next, they investigated its immobilization. Thioxanthone, with functional amide groups as linkages, was immobilized on a modified silica gel. This immobilization not only prevented the leakage of photosensitizer in the solid phase but also enhanced the catalytic activity compared to the parent soluble thioxanthone. This enhancement was particularly interesting, as solid-phase reactions are typically slower than liquid-phase reactions.
This superior catalytic activity was attributed to the introduction of suitable functional groups. The researchers, therefore, evaluated the catalytic activity of various photosensitizers with different functional groups by comparing the total amount of light required for promoting photoisomerization. With the optimal photosensitizer identified, they conducted the photoisomerization reactions in the recycling photoreactor, yielding the desired Z-alkenes in good yields after 4–10 cycles.
“This recycling photoreactor shows promise as an efficient alternative system to produce Z-alkenes,” remarks Prof. Takahashi. “Due to the continuous closed-loop recycling of the samples, it represents an environmentally friendly and sustainable method.”
This innovative method can lead to more eco-friendly development of Z-alkenes, and therefore pharmaceuticals, paving the way towards a sustainable future.
***
Reference
Authors: Mayuko Suga1, Saki Fukushima1, Kosho Makino2, Kayo Nakamura1, Hidetsugu Tabata3, Tetsuta Oshitari3, Hideaki Natsugari4, Noritaka Kuroda5, Kunio Kanemaru6, Yuji Oda6, and Hideyo Takahashi1
Affiliations:
1Faculty of Pharmaceutical Sciences, Tokyo University of Science
2Research Institute of Pharmaceutical Sciences, Musashino University
3Faculty of Pharma Sciences, Teikyo University
4Graduate School of Pharmaceutical Science, The University of Tokyo
5YMC Company Limited
6IWASAKI ELECTRIC CO., LTD.
Media contact
Yoshimasa Iwasaki
Public Relations Division
Tokyo University of Science
Email: mediaoffice@admin.tus.ac.jp
YMC Company Limited
Sales & Marketing Department
Email: support@ymc.co.jp
IWASAKI ELECTRIC CO., LTD.
Public Relations Department
Email: support@iwasaki.co.jp
Funding information
This work was partially supported by A-STEP (JPMJTR213B) from the Japan Science and Technology Agency.
Journal
The Journal of Organic Chemistry
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Isomerization of E‑Cinnamamides into Z‑Cinnamamides Using a Recycling Photoreactor
Article Publication Date
5-Jun-2024
COI Statement
The authors declare no competing financial interest.