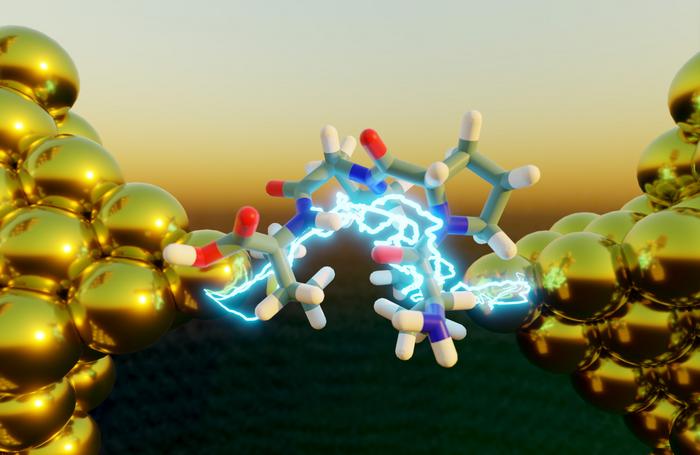
What puts the electronic pep in peptides? A folded structure, according to a new study in the Proceedings of the National Academy of Sciences.

Credit: Moeen Meigooni.
What puts the electronic pep in peptides? A folded structure, according to a new study in the Proceedings of the National Academy of Sciences.
Electron transport, the energy-generating process inside living cells that enables photosynthesis and respiration, is enhanced in peptides with a collapsed, folded structure. Interdisciplinary researchers at the Beckman Institute for Advanced Science and Technology combined single-molecule experiments, molecular dynamics simulations and quantum mechanics to validate their findings.
“This discovery provides a new understanding of how electrons flow through peptides with more complex structures while offering new avenues to design and develop more efficient molecular electronic devices,” said lead investigator Charles Schroeder, the James Economy Professor in Materials Science and Engineering at the University of Illinois Urbana-Champaign.
Proteins reside in all living cells and are integral to cellular activities like photosynthesis, respiration (taking in oxygen and expelling carbon dioxide) and muscle contraction.
Chemically, proteins are long sequences of amino acids strung like holiday lights, the different colors representing different amino acids like tryptophan and glutamine.
In a protein’s simplest form (its primary structure) the amino acid string lies flat. But amino acids are prone to mingling; when they interact with one another, the string tangles, causing the structural collapse referred to as protein folding (or secondary structure).
The researchers asked if and how a protein’s structure impacts its ability to conduct electricity — a question not clearly answered by existing literature.
Rajarshi “Reeju” Samajdar, a graduate student in the Schroeder Group, was patiently probing this protein problem by experimenting on one molecule at a time. But Samajdar was not looking at proteins at all. Instead, he focused on peptides, fragments of proteins with a fraction of the amino acids. For this study, Samajdar used peptides with about four or five amino acids, which permitted more granular observation, he said.
Samajdar saw something surprising: stretched-out peptides with a primary structure seemed to be less effective energy conductors than their folded counterparts with a secondary structure. The stark difference between the peptides’ behavior in each state piqued his curiosity.
“Peptides are very flexible. We were interested in understanding how the conductance properties changed as you stretch them out and the peptides transition from a folded secondary structure to an extended conformation. Interestingly, I saw a distinct jump between those two structures, with different electronic properties in each,” Samajdar said.
To verify his observations, Samajdar called on Moeen Meigooni, a graduate research assistant working with Emad Tajkhorshid, a Beckman researcher, professor and the J. Woodland Hastings Endowed Chair in Biochemistry.
The team simulated the peptides’ conformational behavior with computer modelling, confirming the jerky structural shifts Samajdar observed. Leaving no scientific stones unturned, the researchers worked with Martin Mosquera, an assistant professor of chemistry at Montana State University, and Nicholas Jackson, a Beckman researcher and an assistant professor of chemistry at Illinois, to use quantum mechanical calculations to confirm that these two discrete structures were indeed linked to the changes in conductivity.
“We believe that our approach combining single-molecule experiments, structural modelling with molecular dynamics and quantum mechanics is a very powerful approach for understanding molecular electronics,” Samajdar said. “We could have gone straight to quantum, but we didn’t. The computer simulation piece allowed us to study the entire conformational space of the peptides.”
The researchers’ triple-checked results indicate that peptides with a folded secondary structure do conduct electricity better than peptides with an unfolded primary structure. The specific secondary structure they observed formed a shape called the 310 helix.
Because this work was conducted on peptides, the results lend themselves to a greater understanding of electron transport in larger, more complex proteins and other biomolecules, pointing toward applications in molecular electronic devices like semiconductors that work by switching between two distinct structures.
Editor’s notes:
The publication titled “Secondary structure determines electron transport in peptides” appears in the journal PNAS.
DOI: https://doi.org/10.1073/pnas.2403324121
Contact Charles Schroeder at cms@illinois.edu
Media contact: Jenna Kurtzweil, kurtzwe2@illinois.edu
Emad Tajkhorshid is also affiliated with the Department of Biochemistry, the Neuroscience Program, the Department of Chemistry, the Department of Bioengineering and the Department of Biomedical and Translational Sciences.
Charles Schroeder is also a professor in Chemical and Biomolecular Engineering and is affiliated with the Department of Bioengineering, the Department of Chemistry and the Materials Research Lab.
Nicholas Jackson is also affiliated with the Department of Chemical and Biomolecular Engineering.
For a full list of authors and affiliations, please consult the publication.
Journal
Proceedings of the National Academy of Sciences
Article Title
Secondary structure determines electron transport in peptides
Article Publication Date
25-Jul-2024
COI Statement
Competing interests: The authors declare no competing interest.