A groundbreaking study published in the prestigious journal Cell Reports has illuminated the critical role of insulin-like growth factor (IGF) signaling in the development of zebrafish lymphatic vessels. This investigation offers unprecedented insights into the molecular mechanisms that drive lymphangiogenesis, the process by which lymphatic vessels form and grow. Utilizing the zebrafish model, renowned for its transparent embryonic development and genetic malleability, researchers have visually traced and manipulated the intricate pathways governing lymphatic vasculature formation. These findings not only advance our understanding of vertebrate lymphatic biology but also suggest potential therapeutic avenues for treating lymphoedema, a condition marked by dysfunctional lymphatic drainage and swelling.
Lymphatic vessels play a pivotal role in maintaining fluid homeostasis, immune system function, and fat absorption, yet the precise signaling cascades guiding their embryonic and postnatal formation remain incompletely characterized. The insulin-like growth factor (IGF) pathway, traditionally known for its influence on growth and metabolic regulation, has emerged as a key player in vascular biology. This study bridges an important knowledge gap by conclusively demonstrating that IGF signaling directly regulates the proliferation, migration, and differentiation of lymphatic endothelial cells during vessel morphogenesis.
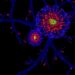
Taking advantage of the zebrafish larval model, scientists engineered fluorescent labeling techniques that distinctly marked lymphatic vessels in red and blood vessels in green. This dual labeling enabled real-time visualization of vessel development within a living organism, an extraordinarily powerful method to dissect the temporal and spatial dynamics of lymphangiogenesis. Through high-resolution confocal imaging paired with genetic and pharmacological manipulation, the researchers unveiled how perturbations in IGF signaling receptors and downstream mediators curtail proper lymphatic vessel growth, resulting in aberrant lymphatic architecture.
The mechanistic heart of the study lies in identifying how IGF interactions activate intracellular pathways that govern cell cycle progression and cytoskeletal reorganization within lymphatic endothelial cells. These biochemical cues are essential for these cells to migrate correctly and form tubular structures. Disruption of IGF signaling was shown to impair the expression of key transcription factors and adhesion molecules necessary for vessel branching and stabilization. Such insights suggest that modulating IGF pathways could effectively promote lymphatic repair or regeneration in disease contexts.
Lymphedema, characterized by localized fluid retention and swelling due to defective lymph drainage, has long been a clinical challenge without effective pharmaceutical interventions. The current research propels the prospect of harnessing molecular drivers like IGF to engineer targeted therapies that stimulate endogenous lymphatic vessel growth or enhance lymphatic function. Zebrafish models provide a compelling platform for screening IGF-targeted compounds and assessing their vascular effects before translation to mammalian studies.
In addition to clarifying the role of IGF in lymphangiogenesis, this work reinforces the sophisticated crosstalk between blood vascular and lymphatic systems. The differential fluorescent labeling highlighted not only individual vessel growth but also the coordinated spatial arrangement influenced by IGF signaling gradients. This coordination is critical for maintaining vascular integrity and preventing pathological leakage or vessel malformations.
The experimental design incorporated loss-of-function and gain-of-function approaches to precisely modulate IGF receptor activity in zebrafish embryos. These manipulations yielded dose-dependent phenotypes, revealing a nuanced regulatory axis wherein optimal levels of IGF signaling are required for proper lymphatic vessel patterning. This dose sensitivity underscores the potential challenges of therapeutic modulation, emphasizing the need for finely tuned interventions.
Further molecular profiling through RNA sequencing identified downstream effectors and gene networks activated by IGF stimulation. These genes encompass those involved in cell adhesion, extracellular matrix remodeling, and angiogenic signaling pathways, collectively contributing to the orchestration of lymphatic vessel morphogenesis. Understanding this transcriptional landscape equips researchers with a broader map of molecular targets to influence lymphatic development.
Moreover, the choice of zebrafish as an experimental organism provided unparalleled advantages in live imaging and rapid genetic testing, accelerating the throughput of discovery compared to traditional mammalian models. The transparency of the zebrafish larvae permitted continuous in vivo observation of vessel dynamics over developmental timeframes, capturing events that static histological techniques cannot resolve.
This research marks a significant advancement in vascular biology by delineating a previously underappreciated functional role of IGF in lymphatic vessel formation. It sets the stage for future inquiries into how metabolic and growth factor signaling networks intersect with vascular development and disease. The translational implications are vast, ranging from innovative treatments for lymphedema to improving outcomes in wound healing and cancer metastasis, where lymphatic vessels play critical roles.
In summary, this landmark study leverages cutting-edge imaging and molecular techniques within the zebrafish model system to reveal that insulin-like growth factor signaling is indispensable for the proper formation of lymphatic vessels. These findings open new vistas in our quest to manipulate lymphangiogenesis for therapeutic benefit, potentially ameliorating conditions rooted in lymphatic dysfunction with precision medicine approaches.
Subject of Research: Cells
Article Title: Insulin-like growth factor signaling regulates zebrafish lymphatic-vessel development
News Publication Date: 24-Feb-2026
Web References: DOI link
Image Credits: University of Auckland
Keywords: Human health, Clinical medicine