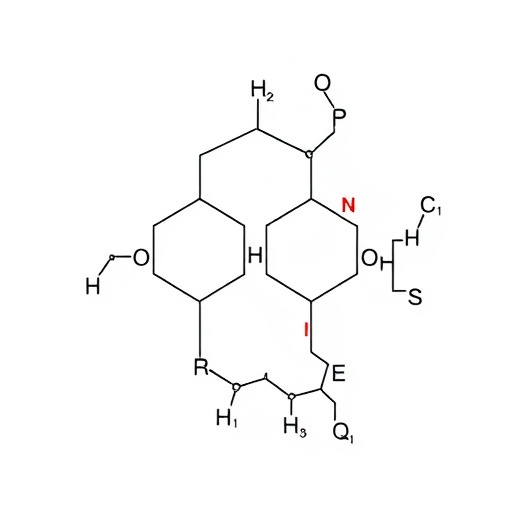
In a groundbreaking advancement that could redefine synthetic methodologies in medicinal chemistry, researchers at the University of Science and Technology of China, spearheaded by Hanmin Huang and Bangkui Yu, have unveiled a highly innovative palladium-catalyzed cascade cyclization process that constructs chiral nitrogen-bridged oxazobicyclic compounds with exceptional stereochemical precision. This pioneering approach strategically harnesses readily available salicylaldehyde and aminodienes as starting materials and ingeniously exploits the in situ formation of three-membered palladium intermediates arising from aldehydes and amines. This novel pathway represents a monumental leap forward from classical synthetic routes that depend heavily on preformed cyclic precursors, often limiting structural diversity and efficiency.
The team’s approach revolves around a seamless, palladium-catalyzed diastereo- and enantioselective sequential cyclization reaction that directly forges complex chiral bridged architectures, overcoming the inherent synthetic challenges posed by topological strain and conformational rigidity common in bridged heterocycles. Unlike traditional methods that struggle to balance yield, diastereoselectivity, and enantioselectivity, this cascade cyclization process consistently delivers bridged oxazole bicyclic frameworks with enantiomeric excesses reaching up to an impressive 96%, coupled with high diastereomeric purity. Such precision is critical, as the three-dimensional configuration of these compounds profoundly influences their biological activity, stability, and pharmacokinetic profiles.
Bridged heterocycles have long been coveted in drug design due to their distinctive three-dimensionality and capacity to engage biological targets with heightened specificity. However, their synthesis is notoriously complicated by the intricate strain energies and spatial constraints introduced by the bridged ring systems, which frequently cause suboptimal yields and poor stereocontrol. Most conventional syntheses require laborious preparation of cyclic intermediates, severely limiting the versatility of accessible scaffolds. The catalytic strategy devised by Huang’s group circumvents these pitfalls by initiating the cascade from simple acyclic building blocks, vastly expanding the accessible chemical space of chiral bridged nitrogen heterocycles.
Central to the breakthrough is the palladium catalyst’s ability to generate reactive three-membered ring palladium intermediates in situ through condensation of aldehydes and amines, a step that forms the cornerstone for subsequent selective cyclizations. This unique reactivity has permitted the continuous formation of bridged ring systems in a one-pot fashion, streamlining synthesis and mitigating the need for intermediate isolations or chiral auxiliaries. This elegantly orchestrated cascade embodies an exquisite balance of kinetics and thermodynamics controlled by subtle ligand and reaction condition tuning, achieving remarkable control over stereochemical outcomes.
Moreover, this method demonstrates formidable substrate scope, accommodating a broad spectrum of substituted salicylaldehydes and aminodienes without sacrificing stereoselectivity or yield. This versatility not only affirms the robustness of the catalytic system but also facilitates access to a diverse array of bridged heterocyclic structures, significantly enriching the chemical library available for pharmaceutical exploration. The structural diversity attainable via this platform offers medicinal chemists new scaffolds for probing structure-activity relationships and optimizing lead compounds for central nervous system indication and beyond.
Importantly, the synthetic potential of these chiral bridged oxazobicycles extends beyond their initial formation. The research team showcased the facile transformation of these scaffolds into spirocyclic frameworks—an increasingly important motif in drug discovery due to its unique conformational rigidity and biological implications—through strategic chiral transfer methodologies. This adaptability not only underscores the utility of the synthesized frameworks as versatile intermediates but also hints at their potential role in the late-stage diversification of pharmacophores.
Additionally, the inherent chemical reactivity of the formed compounds enables a wide range of post-cyclization functionalizations. Capitalizing on the presence of unsaturated carbon-carbon bonds within the bridged ring systems, the researchers demonstrated efficient derivatization reactions, including epoxidation and hydroboration-oxidation. Such transformations open avenues for further molecular complexity enhancement, tailored functionality, and the design of novel drug candidates with optimized physicochemical properties, thereby broadening the practical scope and applicability of the initial synthetic approach.
This study not only propels the field of asymmetric synthesis forward by delivering an unprecedented, modular, and efficient construction of chiral bridged nitrogen heterocycles but also provides vital mechanistic insights. Understanding the delicate interplay of palladium catalysis, intermediate formation, and stereocontrol within this cascade cyclization informs future catalyst and reaction system design, enabling broader applications and improved synthetic performance for other challenging molecular architectures.
Looking ahead, the catalytic platform developed here holds significant promise for application in complex natural product synthesis and the expedited discovery of lead compounds in medicinal chemistry. The ability to rapidly assemble three-dimensional, chiral, bridged frameworks with high stereochemical fidelity is anticipated to accelerate synthetic campaigns aimed at enriching molecular libraries with structurally sophisticated and biologically relevant entities. Such advancements are vital to overcoming current limitations in drug development pipelines, particularly for central nervous system disorders and other therapeutic areas requiring precise molecular configurations.
This robust and innovative methodology demonstrates a visionary synthesis strategy that aligns with the increasing demand for efficient, selective, and sustainable synthetic transformations. The transition from relying on preformed cyclic precursors to the direct construction of intricate bridged systems from simple acyclic units epitomizes a paradigm shift in synthetic organic chemistry, poised to influence both academic research and industrial drug discovery workflows substantially.
The findings of this research, published as an open-access Communication in the flagship journal CCS Chemistry of the Chinese Chemical Society, exemplify the high-impact innovations emerging from Chinese scientific institutions in contemporary catalysis and drug synthesis. Supported by major national scientific programs and foundations, this work reflects a cohesive effort to tackle some of the most challenging aspects of asymmetric synthesis through meticulous mechanistic exploration and inventive catalyst design.
The research not only expands the frontiers of chiral bridged heterocycle synthesis but also serves as a testament to the increasingly globalized and interdisciplinary nature of modern chemical sciences. Through integrating organometallic catalysis, molecular design, and synthetic strategy, the study lays a robust foundation for next-generation methodologies that combine efficiency, selectivity, and functional versatility, ultimately shaping the future landscape of chemical synthesis and drug development.
Subject of Research: Not applicable
Article Title: Modular Assembly of Chiral Bridged Oxazobicycles via Palladium-Catalyzed Diastereo- and Enantioselective Sequential Cyclization
News Publication Date: 1-Jan-2026
Image Credits: Credit: CCS Chemistry
Keywords
Asymmetric synthesis