In a groundbreaking study published in Nature Communications, researchers have unveiled critical insights into the molecular mechanisms underlying vascular repair and homeostasis following neonatal hyperoxic lung injury. The team, led by Sun, Zhao, Do, and colleagues, has identified the transcription factor FOXF2 as a pivotal regulator of pericyte-endothelial cell communication, essential for maintaining vascular integrity after the damaging effects of excessive oxygen exposure in newborn lungs. This discovery not only enhances our understanding of lung vascular biology but also holds promising implications for therapeutic strategies aimed at mitigating chronic lung diseases in neonates.
Neonatal hyperoxic lung injury remains a significant clinical concern, especially in premature infants requiring supplemental oxygen therapy. While oxygen is lifesaving, prolonged exposure to high oxygen concentrations can induce structural and functional damage to the delicate pulmonary vasculature. The lung’s microvascular network, composed primarily of endothelial cells and pericytes, plays a vital role in sustaining tissue oxygenation and vascular stability. Disruptions in the crosstalk between these cell types can precipitate long-term vascular abnormalities and contribute to conditions such as bronchopulmonary dysplasia (BPD).
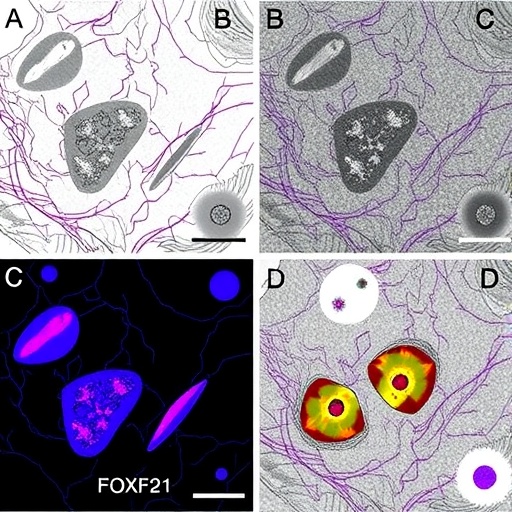
The study delves deeply into the transcriptional regulatory landscape governing pericytes, specialized mural cells closely associated with the capillary endothelium. Through a combination of genetic models, molecular assays, and advanced imaging techniques, the researchers delineate how FOXF2 orchestrates gene expression programs critical for pericyte functionality. FOXF2 emerges as a master regulator that modulates signaling pathways facilitating communication between pericytes and endothelial cells, a dialogue essential for vascular remodeling and repair after injury.
One of the remarkable findings is that FOXF2 expression is significantly upregulated in pericytes following hyperoxic injury, suggesting an adaptive response mechanism. Loss-of-function experiments revealed that depleting FOXF2 impairs pericyte ability to support endothelial cells, leading to compromised vascular barrier integrity and aberrant vessel formation. Such disruptions manifested as increased vascular leakage and insufficient vascular maturation, underscoring the transcription factor’s indispensable role in preserving pulmonary microvascular homeostasis during oxidative stress.
The molecular mechanisms by which FOXF2 exerts its effects involve the modulation of key signaling pathways, including PDGF-BB/PDGFRβ and TGF-β signaling. These pathways are critical in regulating pericyte proliferation, migration, and attachment to endothelial cells. The investigators demonstrated that FOXF2 directly binds promoter regions of genes within these pathways, fine-tuning their activity to maintain balance between vessel stabilization and remodeling. This regulatory axis forms the foundation for effective vascular regeneration after neonatal lung injury.
Importantly, the study employed a neonatal mouse model exposed to hyperoxia to recapitulate aspects of human neonatal lung injury. Using lineage tracing and single-cell RNA sequencing, the researchers characterized pericyte heterogeneity and observed that FOXF2-positive pericyte subpopulations expanded preferentially after hyperoxic challenge. These pericytes displayed distinct transcriptional profiles promoting angiogenesis and extracellular matrix remodeling, highlighting the complexity of cellular responses orchestrated by FOXF2 under stress conditions.
The pathophysiological relevance of these findings extends beyond developmental lung disorders. The principles uncovered regarding FOXF2-mediated pericyte-endothelial crosstalk may inform therapeutic interventions for a broad spectrum of vascular diseases where endothelial dysfunction and pericyte loss are prominent features. By targeting FOXF2 pathways, future treatments might enhance vascular repair mechanisms, potentially reversing or mitigating the damage inflicted by oxidative or inflammatory insults.
Advanced imaging approaches provided compelling visual evidence of how loss of FOXF2 disrupts vascular architecture. Confocal microscopy showed irregular capillary networks with reduced pericyte coverage in FOXF2-deficient lungs. These structural changes correlated strongly with platelet-endothelial cell adhesion molecule (PECAM) staining patterns, confirming endothelial destabilization. Such phenotypic alterations underscore the importance of transcriptional control in vascular cell interplay and structural integrity under injury conditions.
In addition to functional and structural analyses, the research team explored potential downstream effectors regulated by FOXF2. They identified several candidate molecules involved in cytoskeletal dynamics, cell adhesion, and extracellular matrix interactions that contribute to the mechanical and signaling functions of pericytes. These effectors provide a nuanced understanding of how transcriptional regulation translates into cellular behaviors imperative for vascular maintenance.
Clinical implications of these insights are profound. Neonates suffering from hyperoxia-induced lung damage often experience persistent respiratory difficulties and vascular abnormalities that compromise long-term health outcomes. Interventions that bolster endogenous reparative pathways via FOXF2 activation or mimicry could revolutionize neonatal care by reducing complications associated with oxygen therapy. Moreover, understanding FOXF2’s role could aid in the design of biomarkers to predict disease progression or therapeutic responses.
The study also provides a framework for exploring similar transcriptional regulators in other organ systems where pericytes and endothelial cells collaborate to form intricate vascular networks. Given the universal importance of pericyte-endothelial signaling in tissue homeostasis, the principles revealed by this work may apply to pathologies ranging from diabetic retinopathy to cerebral small vessel disease. The versatility of FOXF2’s regulatory capacity could represent a unifying theme in vascular biology.
This landmark research signifies a leap forward in unraveling the molecular dialogue essential to vascular homeostasis post-injury. By illuminating the role of FOXF2 as a guardian of pericyte function and vascular integrity, it opens new avenues for precision medicine aimed at protecting and restoring microvascular networks in vulnerable patient populations. The implication that transcription factor modulation can recalibrate complex intercellular signaling holds exciting promise for future therapeutic development.
As research advances, the integration of FOXF2-related findings with emerging technologies such as gene editing and bioengineered lung scaffolds may further enhance repair strategies. The possibility of manipulating pericyte dynamics to optimize endothelial support could redefine how clinicians approach diseases characterized by vascular instability. This convergence of molecular biology and translational medicine exemplifies the potential of targeted interventions informed by detailed mechanistic insights.
Future investigations will be crucial to fully delineate the upstream regulators of FOXF2 expression and its interaction with other transcriptional networks in the lung microenvironment. Understanding these layers of regulation will provide a more comprehensive picture of vascular adaptation to injury and stress. Such knowledge is vital for developing combination therapies that leverage multiple pathways to achieve sustained vascular repair.
Intriguingly, the study’s findings suggest that FOXF2 could also influence immune-endothelial-pericyte interactions, given the role of vascular cells in inflammation and immunity. Elucidating these relationships may expand the therapeutic relevance of FOXF2 beyond structural maintenance to include modulation of immune responses in injured lungs. This broader scope positions FOXF2 as a multifunctional regulator essential for holistic lung recovery.
In summary, the work by Sun, Zhao, Do, and colleagues heralds a new chapter in vascular biology by defining FOXF2 as an essential transcriptional hub coordinating pericyte-endothelial signaling for vascular homeostasis after neonatal hyperoxic lung injury. This pioneering discovery not only enriches our mechanistic understanding but also sets the stage for innovative interventions designed to protect and restore lung vasculature in the most vulnerable patients, paving the way toward improved clinical outcomes and enhanced quality of life for affected neonates.
Subject of Research: Regulation of pericyte-endothelial signaling by FOXF2 in vascular homeostasis following neonatal hyperoxic lung injury.
Article Title: FOXF2 regulates pericyte–endothelial signaling required for vascular homeostasis after neonatal hyperoxic lung injury.
Article References:
Sun, F., Zhao, Y., Do, J. et al. FOXF2 regulates pericyte–endothelial signaling required for vascular homeostasis after neonatal hyperoxic lung injury. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69525-7
Image Credits: AI Generated