Researchers have uncovered how a structure in bodily carbohydrates (sugar chains or “glycans”) that regulates how they attach themselves to other molecules interacts with key enzymes, and in so doing can contribute to a range of diseases.

Credit: Yasuhiko Kizuka, iGCORE
Researchers have uncovered how a structure in bodily carbohydrates (sugar chains or “glycans”) that regulates how they attach themselves to other molecules interacts with key enzymes, and in so doing can contribute to a range of diseases.
One of the most essential bodily biochemical processes involves carbohydrates (sugan chains or “glycans”) attaching themselves to proteins and fats (lipids), and when this process malfunctions, the risk of contracting a raft of diseases sharply increases. Researchers have recently discovered how a crucial enzyme’s interaction with a small structure in glycans during this attachment can contribute to such breakdowns.
Their findings are described in a paper published online in the Journal of Biological Chemistry on June 4th, 2024.
Within organisms, the attachment of carbohydrates, or “glycans”, onto proteins or lipids—a process called “glycosylation”—plays an essential role in a staggering number of physiological processes. It is necessary for cell recognition, cell signaling, immune response, protein folding, development and fertilization. Meanwhile, the slightest alteration in the structure of glycans can lead to or aggravate diseases from cancer and diabetes to Alzheimer’s and muscular dystrophy.
Glycans and their associated processes are in fact so important that they get their own field: glycobiology. And within this discipline, almost all of the enzymes—the molecules that kick off or speed up chemical reactions—that are responsible for production of glycans in humans have been identified and categorized, as well as the various production processes, or ‘biosynthetic pathways.’
However, despite the maturity of glycobiology as a field, researchers and clinicians need to be able to more precisely predict and fine-tune the glycan structures in cells—and thence better respond to the various diseases that malfunctioning glycosylation can cause. As a result, researchers still need to better understand how the activity of the enzymes involved in attaching glycans to the proteins or lipids—“glycotransferases”—is regulated within cells.
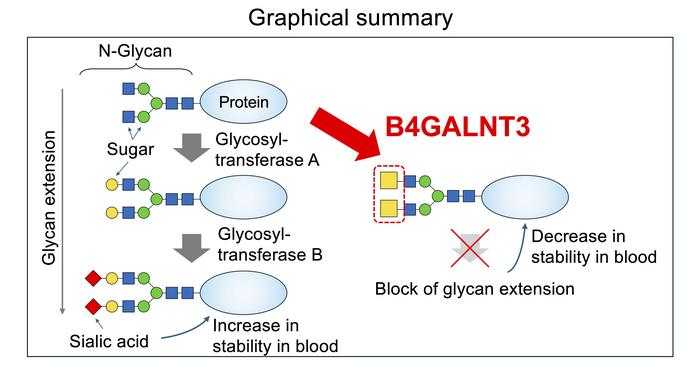
Within two types of glycans, N-linked glycans (those glycans attached to a nitrogen atom within proteins), and O-linked glycans (those attached to an oxygen atom within proteins), there is a structure made up of two sugars called LacdiNAc, or just LDN, present at the end (terminal position) or semi-end (sub-terminal position) of the glycan. The biosynthesis of the LDN structure is catalyzed by one of two enzymes, B4GALNT3 or B4GALNT4. In other glycans, there is no LDN, but instead a slightly different two-sugar structure called LacNAc.
This LDN structure has been shown to be involved in a range of important bodily processes, and its malfunctioning in many diseases. In particular, several hormones that circulate in the body are modified by LDN, and LDN appears to be involved in maintenance of stem cells. In addition, variants of the B4GALNT3 that catalyses LDN biosynthesis have been associated with lower bone density and higher risk of fractures, and when B4GALNT3 appears to be turned off, risk of colon cancer is increased.
“Given all these smoking guns related to disease and key bodily processes, it’s vital that we understand how these enzymes synthesize LDN,” said Yasuhiko Kizuka, lead researcher on the paper and a glycobiology with the Institute for Glyco-core Research (iGCORE) at Gifu University. “But until now, these processes have been something of a black box.”
But in recent years, advances in structural biology, enzymology, and glycomics have delivered new tools and techniques that open up the possibility of such investigations.
First, by screening structures of all known human glycosyltransferases, the researchers found that in both types of B4GALNT, there is a unique feature, the “PA14 domain” not commonly found in other glycosyltransferases.
The researchers also compared the enzymatic activity of normal B4GALNT3 with versions of the glycosyltransferase that they had mutated so that it lacked the PA14 domain to see what happens when this structure is missing. Then, they compared the activity of normal B4GALNT3 with other normal glycosyltransferases and glycan-modifying enzymes.
In addition, the authors of the paper produced computer models of both B4GALNT3 and its PA14 domain to investigate the potential binding interactions and structural features that influence enzyme activity. They also performed various biochemical analyses to characterize the structures of the glycans produced by B4GALNT3, including their “epitopes”—the recognition site for interaction with other molecules—and then investigated the effects of LDN on the manufacture of these epitopes.
They found that mutated versions of B4GALNT3 lacking the PA14 domain suffered from dramatically decreased activity relating to N-linked and O-linked glycans, as well as to glycoproteins. This suggests that the PA14 domain is essential for enzyme activity.
The researchers were also able to better understand the functional differences between LDN and LacNAc. They discovered that the presence of LDN in place of LacNAc had a negative impact on the actions of many glycosyltransferases in modification or addition of sugar residues at the terminal ends of N-glycans. These terminal modifications—called “N-glycan capping”—play a crucial role in determining the structure, function, stability and interactions of glycoproteins.
“In other words, LDN has significant impacts on the production of N-glycans that are completely different from the typical LacNAc structure,” said Yasuhiko Kizuka.
The researchers now hope to delve deeper into the role of B4GALNT3 and LDN in disease pathogenesis and identification of potential therapeutic targets.
Journal
Journal of Biological Chemistry
Method of Research
Experimental study
Subject of Research
Cells
Article Title
LacdiNAc synthase B4GALNT3 has a unique PA14 domain and suppresses N-glycan capping
Article Publication Date
4-Jun-2024