In a groundbreaking study set to reshape clinical approaches to infectious diseases, researchers have demonstrated that simultaneous treatment of tuberculosis (TB) and human immunodeficiency virus (HIV) infections effectively suppresses TB reactivation during co-infection but fails to mitigate the chronic immune activation that plagues many patients. This new evidence, published in Nature Communications in 2025, sheds light on the intricate immunological interplay between these two deadly pathogens and challenges existing therapeutic paradigms.
The dual burden of TB and HIV continues to pose a formidable public health challenge globally. HIV-infected individuals are significantly more susceptible to developing active TB, with co-infection driving morbidity and mortality rates higher than either infection alone. Until now, standard clinical practices have emphasized concurrent antiretroviral therapy (ART) alongside anti-TB treatment to manage both diseases effectively. However, the immunopathological consequences of this co-treatment strategy have remained somewhat obscure.
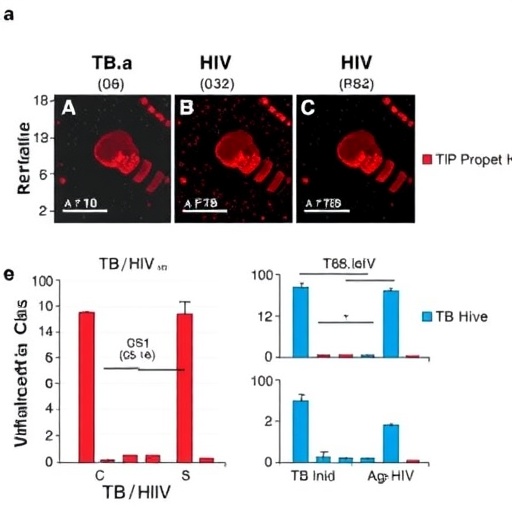
Led by Sharan, Zou, Singh, and colleagues, this study undertook an exhaustive analysis of immune responses during co-infection treatments to decipher why, despite successful control of TB reactivation, persistent immune activation remains unresolved. Employing a combination of longitudinal clinical data and sophisticated immunological assays, the team delved into cellular and molecular signatures that underlie chronic inflammation, a key driver of disease progression and patient deterioration.
Their findings reveal that while combined therapy markedly reduces active TB episodes by suppressing Mycobacterium tuberculosis replication, it does not sufficiently restore immune homeostasis. Notably, markers of systemic immune activation—such as elevated pro-inflammatory cytokines, persistent T-cell activation, and increased monocyte turnover—continue unabated, pointing to a complex immunological milieu unresponsive to existing treatment regimens.
One pivotal discovery relates to the role of HIV-induced immune dysregulation that outlasts viral suppression. Even with effective ART reducing HIV viral loads to undetectable levels, the residual activation of innate and adaptive immune cells perpetuates a state of chronic inflammation. This persistent activation carries significant consequences, including heightened risk for cardiovascular disease, neurocognitive decline, and other non-AIDS-defining comorbidities which compound patient health vulnerabilities.
The study employed advanced flow cytometry and transcriptomic profiling to capture the nuanced immune cell dynamics throughout therapy. Crucially, the researchers identified specific subsets of activated T cells and inflammatory monocytes that are refractory to both anti-TB and antiretroviral interventions. These cell populations likely sustain the inflammatory environment, perpetuating tissue damage and systemic immune exhaustion despite pathogen control.
Furthermore, the investigation illuminates the challenge of timing and integration in dual therapy. Early intervention appears critical in limiting TB reactivation; however, the persistence of immune activation suggests that current therapeutic windows might not adequately address the restoration of immune regulation. The interplay between microbial suppression and immune recalibration requires more refined approaches that transcend conventional antimicrobial treatments.
The implications of this research are profound for clinical management. It suggests that adjunctive therapies targeting immune activation—such as anti-inflammatory agents or immune modulators—may be necessary to complement pathogen-directed treatments. Future therapeutic frameworks might integrate tailored immunomodulation to enhance patient outcomes by mitigating collateral immune damage without compromising the efficacy of infection control.
From a broader scientific perspective, this study advances our understanding of pathogenic synergy and immune pathophysiology during co-infection. It underscores the necessity of considering host immune dynamics as integral to treatment success rather than focusing solely on pathogen eradication. The concept of “pathogen control without immune restoration” emerges as a paradox that could explain the discrepancies between microbial suppression and clinical recovery witnessed in many co-infected patients.
Moreover, the research highlights potential biomarkers for monitoring immune activation beyond viral load and TB culture positivity. These biomarkers could inform personalized therapeutic adjustments, optimizing timing and selection of adjunctive treatments. The identification of immune signatures resistant to standard therapy opens avenues for novel drug discovery aimed at curbing chronic inflammation and associated comorbidities.
In clinical practice, the findings advocate for heightened vigilance in managing co-infected individuals even after achieving microbiological remission. Regular assessment of immune activation markers could become standard to preempt long-term complications. Additionally, patient education must evolve to encompass the understanding that infection control does not equate to immunological normalization, underscoring the importance of continuous monitoring and care.
The study also poses critical questions regarding the mechanisms sustaining chronic immune activation during suppressed viral replication. Whether residual viral reservoirs, microbial translocation from the gut, or persistent antigenic stimulation from latent TB drives this inflammation remains an area for further exploration. Understanding these mechanisms will be pivotal in designing interventions that can break the cycle of immune activation.
In essence, Sharan and colleagues have beautifully dissected the complex landscape of TB-HIV co-infection treatment, revealing a scenario where current therapies are necessary but insufficient. Their work propels the field toward a more nuanced paradigm—one that simultaneously controls pathogens, reprograms immune responses, and ultimately improves clinical outcomes for millions affected worldwide.
As the global health community grapples with the persistent dual epidemics of TB and HIV, these insights offer a beacon for future research and clinical strategies. Tailored immunotherapeutic interventions integrated into comprehensive treatment plans might finally bridge the gap between infection control and immune restoration. The path forward calls for interdisciplinary collaboration, combining immunology, infectious disease expertise, and pharmacology to conquer the intertwined threats of TB and HIV.
This seminal publication not only enhances scientific knowledge but also redefines expectations for disease management in co-infected populations. It paves the way for innovative trials and policy frameworks that could dramatically reduce the burden of chronic immune activation-related complications, heralding a new era in infectious disease therapeutics.
Subject of Research: The study investigates the effects of concurrent tuberculosis and HIV therapies on TB reactivation control and chronic immune activation during co-infection.
Article Title: Concurrent TB and HIV therapies control TB reactivation during co-infection but not chronic immune activation.
Article References:
Sharan, R., Zou, Y., Singh, B. et al. Concurrent TB and HIV therapies control TB reactivation during co-infection but not chronic immune activation. Nat Commun (2025). https://doi.org/10.1038/s41467-025-67188-4
Image Credits: AI Generated