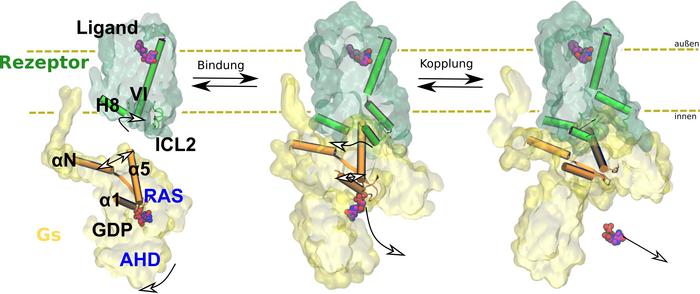
Every organism reacts to its environment. An external stimulus causes the body to release messengers such as adrenaline, which bind to receptors. The receptors transmit the signal to other proteins. This triggers biochemical cascades that lead to a response in the organism, such as a flight-or-fight response in case of the adrenaline-binding receptor. Drugs are often modelled based on these messengers and work by interacting with receptors. Side effects can occur if the drug binds to the wrong receptor or does not transmit the signal to the correct intracellular protein. To prevent this, scientists are studying how receptors work. In the current study, Professor Peter Hildebrand and his team from the Institute of Medical Physics and Biophysics at Leipzig University show how signal transmission through the β2 adrenergic receptor takes place at the atomic level. This is a G protein-coupled receptor (GPCR). The members of this protein superfamily are embedded in the cell membrane.
The team used computer-aided molecular dynamics simulations as well as biochemical and functional mutation analyses for their investigations. This allowed them to observe how the receptor works: by binding, the receptor changes the three-dimensional structure of intracellular G protein, which then releases the regulatory molecule GDP. In the next step, this G protein can be activated by binding its actual substrate GTP and trigger biochemical cascades in the cell. The team of researchers also found that the exact function of the receptor depends on the arrangement of various flexible structural elements. They cannot be characterised using classical structural biology methods.
Professor Hildebrand is now planning to apply the computer-aided biophysical methods to other receptor systems, such as in obesity research, a focus of medical research at Leipzig University. “Comparative studies of dynamic signalling are exciting when drugs with different profiles are used,” explains the professor of biophysical computer simulations.
Professor Peter W. Hildebrand has been researching receptors at the Faculty of Medicine at Leipzig University since 2017. From 2008–2014, he studied the structure of the photoreceptor rhodopsin with Professor Klaus-Peter Hofmann and Dr Patrick Scheerer at the Charité. He is now also collaborating with Nobel laureate Professor Brian Kobilka and cryo-electron microscopist Professor Yiorgo Skiniotis, Stanford University, US, to better understand GPCR-mediated signalling. Together, they recently elucidated the mechanism of GTP binding to the G protein and its activation, and published the results in Nature. “For the first time, we now have a comprehensive picture of the structural mechanism of receptor-mediated signalling from the outside to the inside of the cell,” says Hildebrand, summarising his research. “Alongside my collaborators, I owe this success above all to the talented young scientists Dr Hossein Batebi and Dr Guillermo Pérez-Hernández from my team.” At Leipzig University, G protein-coupled receptors are also the focus of Collaborative Research Centre (CRC) 1423, Structural Dynamics of GPCR Activation and Signaling, which is led by Professor Annette Beck-Sickinger.

Credit: Photo: Peter W. Hildebrand
Every organism reacts to its environment. An external stimulus causes the body to release messengers such as adrenaline, which bind to receptors. The receptors transmit the signal to other proteins. This triggers biochemical cascades that lead to a response in the organism, such as a flight-or-fight response in case of the adrenaline-binding receptor. Drugs are often modelled based on these messengers and work by interacting with receptors. Side effects can occur if the drug binds to the wrong receptor or does not transmit the signal to the correct intracellular protein. To prevent this, scientists are studying how receptors work. In the current study, Professor Peter Hildebrand and his team from the Institute of Medical Physics and Biophysics at Leipzig University show how signal transmission through the β2 adrenergic receptor takes place at the atomic level. This is a G protein-coupled receptor (GPCR). The members of this protein superfamily are embedded in the cell membrane.
The team used computer-aided molecular dynamics simulations as well as biochemical and functional mutation analyses for their investigations. This allowed them to observe how the receptor works: by binding, the receptor changes the three-dimensional structure of intracellular G protein, which then releases the regulatory molecule GDP. In the next step, this G protein can be activated by binding its actual substrate GTP and trigger biochemical cascades in the cell. The team of researchers also found that the exact function of the receptor depends on the arrangement of various flexible structural elements. They cannot be characterised using classical structural biology methods.
Professor Hildebrand is now planning to apply the computer-aided biophysical methods to other receptor systems, such as in obesity research, a focus of medical research at Leipzig University. “Comparative studies of dynamic signalling are exciting when drugs with different profiles are used,” explains the professor of biophysical computer simulations.
Professor Peter W. Hildebrand has been researching receptors at the Faculty of Medicine at Leipzig University since 2017. From 2008–2014, he studied the structure of the photoreceptor rhodopsin with Professor Klaus-Peter Hofmann and Dr Patrick Scheerer at the Charité. He is now also collaborating with Nobel laureate Professor Brian Kobilka and cryo-electron microscopist Professor Yiorgo Skiniotis, Stanford University, US, to better understand GPCR-mediated signalling. Together, they recently elucidated the mechanism of GTP binding to the G protein and its activation, and published the results in Nature. “For the first time, we now have a comprehensive picture of the structural mechanism of receptor-mediated signalling from the outside to the inside of the cell,” says Hildebrand, summarising his research. “Alongside my collaborators, I owe this success above all to the talented young scientists Dr Hossein Batebi and Dr Guillermo Pérez-Hernández from my team.” At Leipzig University, G protein-coupled receptors are also the focus of Collaborative Research Centre (CRC) 1423, Structural Dynamics of GPCR Activation and Signaling, which is led by Professor Annette Beck-Sickinger.
Tom Goetze
Journal
Nature Structural & Molecular Biology
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Mechanistic insights into G-protein coupling with an agonist-bound G-protein-coupled receptor
Article Publication Date
12-Jun-2024