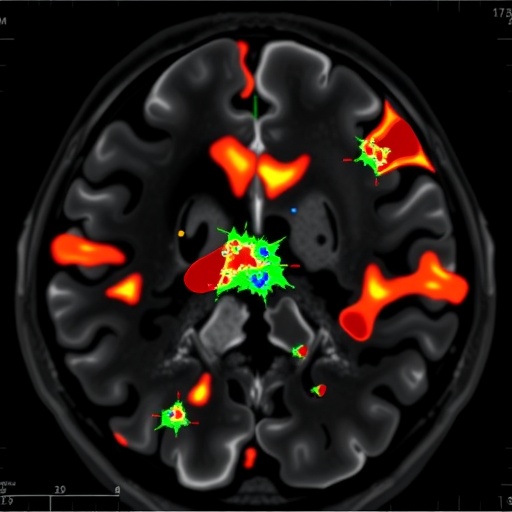
In a groundbreaking study published in the Journal of Medical Biologt Engineering, researchers have taken a significant leap forward in the automated detection and segmentation of cerebral arteriovenous malformations (AVMs) utilizing advanced Gaussian Mixture Model (GMM) clustering techniques. The significance of this research cannot be overstated, as AVMs represent a critical concern in neurovascular pathology, often leading to severe neurological deficits and considerable morbidity in affected individuals. Traditional methods of identifying and mapping these complex vascular anomalies are often labor-intensive and fraught with diagnostic inaccuracies. The innovative approach outlined by the authors, Lin, Lee, and Chen et al., promises to offer a robust alternative that enhances both efficiency and precision in clinical settings.
Cerebral AVMs, characterized by a tangle of abnormal blood vessels connecting arteries and veins, pose a substantial risk of hemorrhagic strokes. As a result, accurately identifying these lesions is fundamental to ensuring proper treatment strategies, which may involve surgical intervention or radiological therapies. The ability to segregate these pathological formations with a high degree of accuracy is imperative to reducing the incidence of misdiagnosis that can lead to devastating consequences for patients. With their study, the authors explore the efficacy of Gaussian Mixture Model clustering as a solution to the inherent challenges associated with traditional diagnostic methodologies.
Gaussian Mixture Models are probabilistic models that assume all data points are generated from a mixture of several underlying Gaussian distributions. By leveraging this statistical approach, the researchers developed an automated segmentation framework specifically geared towards magnetic resonance imaging (MRI) data. This methodology is particularly well-suited for medical imaging applications due to its ability to handle noise and variance in data effectively. As a result, implementing GMM allows for a refined analysis of the complex structures associated with AVMs in MRIs.
A key feature of this study is the vast dataset that the researchers utilized, which comprised numerous MRIs with confirmed AVM pathology. The diversity within this dataset enabled the GMM to learn and adapt to variations in morphology and size of AVMs, facilitating segmentation accuracy across a wide range of clinical scenarios. The results were promising, demonstrating not only the reliability of the model in identifying AVMs but also its potential application in real-time settings.
One of the standout aspects of the research is the incorporation of machine learning techniques into the segmentation process. By training the GMM with labeled data, the model was able to improve its predictive capabilities, making the segmentation process progressively more precise. Through iterative learning and refinement, the researchers achieved a model that could effectively decrease false positives and negatives in segmentation, thereby enhancing overall diagnostic accuracy.
In addition to the statistical modeling aspect, the authors paid close attention to the preprocessing of MRI images, which plays a crucial role in the effectiveness of image analysis algorithms. The researchers employed advanced techniques to denoise the MRI data and normalize the image intensities. Such preprocessing steps are not just procedural; they lay the foundation for the GMM to perform optimally and achieve the desired segmentation outcomes.
Another noteworthy element of this study is the comparison of the GMM clustering results with traditional segmentation methods, such as thresholding and region-growing techniques. The authors found that their Gaussian Mixture Model markedly outperformed these standard methods, displaying resilience in the face of irregularities and artifacts often present in clinical images. The comparative analysis not only highlights the capabilities of the GMM approach but also reinforces the need for integrating more sophisticated algorithms in medical imaging.
A comprehensive performance evaluation was conducted through metrics such as the Dice Similarity Coefficient (DSC) and Jaccard Index to quantify segmentation accuracy. Results indicated that the GMM model achieved a higher DSC than traditional methods, which is critical for clinicians who depend on accurate depictions of vascular anomalies to guide treatment decisions. These findings underscore the potential for GMM clustering to revolutionize AVM evaluation and treatment planning, positively impacting patient outcomes.
Notably, the research does not merely present a theoretical model; it also paves the way for further exploration into the integration of artificial intelligence in medical imaging. The implications of such advancements are vast, as they offer the prospect of automated diagnoses that lessen the burden on healthcare professionals. In the era where time is often of the essence in medical scenarios, expedited and accurate diagnosis could lead to earlier intervention and, ultimately, improved patient prognoses.
Moreover, the relevance of this study extends beyond AVMs alone. The methodologies and frameworks established through the use of Gaussian Mixture Models could easily be adapted to other medical imaging challenges, including the identification of tumors or other vascular irregularities. This versatility adds yet another layer of significance to the researchers’ work, highlighting the potential for standardized approaches across various medical fields.
As medical imaging technology continues to evolve, the contributions of studies like this one serve as a catalyst for innovative practices. By establishing a free-flowing synergy between advanced machine learning techniques and clinical application, the research team is setting a benchmark for future investigations into automated medical diagnostics. It is anticipated that as technology progresses, such methodologies will become increasingly integrated into routine clinical practice, ultimately leading to a new era of precision medicine.
As we reflect on the implications of this research, it becomes apparent that the integration of sophisticated algorithms like the Gaussian Mixture Model necessitates a collaborative approach across disciplines. Clinicians, data scientists, and researchers must work in concert to ensure that such technologies are effectively harnessed, paving the way for research that not only advances technical capabilities but also aligns with the evolving landscape of patient care.
In conclusion, the work spearheaded by Lin, Lee, and Chen et al. marks a pivotal moment in the automation of medical imaging. By employing Gaussian Mixture Model clustering for the segmentation of cerebral AVMs, the authors have created a promising avenue for improving diagnostic accuracy and efficiency in the medical field. This approach not only represents a significant advancement in the identification and understanding of complex neurovascular conditions but also stands as an exemplar for the integration of modern computational techniques in healthcare. As we move forward, the imperative to embrace such innovations in medicine continues to grow, pointing to a future where automated diagnostic procedures could redefine patient experience and outcomes.
Subject of Research: Automated Segmentation of Cerebral Arteriovenous Malformations in Magnetic Resonance Images
Article Title: Using Gaussian Mixture Model Clustering for the Automated Segmentation of Cerebral Arteriovenous Malformations in Magnetic Resonance Images
Article References:
Lin, TY., Lee, C., Chen, YW. et al. Using Gaussian Mixture Model Clustering for the Automated Segmentation of Cerebral Arteriovenous Malformations in Magnetic Resonance Images.
J. Med. Biol. Eng. 45, 13–21 (2025). https://doi.org/10.1007/s40846-024-00919-y
Image Credits: AI Generated
DOI:
Keywords: Cerebral arteriovenous malformations, Gaussian Mixture Model, automated segmentation, medical imaging, machine learning