A recent study published in the journal “Gene Therapy” by Stamataki et al. sheds light on the advancements in the field of gene therapy, specifically focusing on adeno-associated virus (AAV) variants. This groundbreaking research aims to refine the transduction efficiency of these viral vectors, particularly in human vascular endothelial cells, a critical target for various therapeutic applications. The study utilizes sophisticated AAV capsid libraries screened in non-human primates, paving the way for enhanced gene delivery mechanisms that could drastically improve treatment outcomes for cardiovascular diseases and other conditions.
The significance of AAVs in gene therapy cannot be overstated; these viruses are notably non-pathogenic and possess a remarkable ability to transduce a range of cell types. Their unique properties make them a staple in delivering genetic material, making them valuable tools in genetic research and clinical applications. The primary goal of Stamataki’s study was to identify AAV variants that exhibit superior transduction capabilities, enhancing their effectiveness in therapeutic settings involving human vascular endothelial cells.
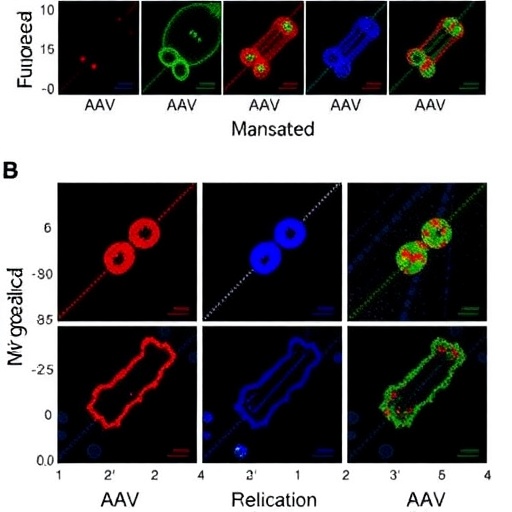
One of the critical challenges in gene therapy is achieving efficient and targeted delivery of therapeutic genes to the desired cells. Traditional methods often fall short due to limitations in the natural tropism of AAVs, which necessitates the exploration of modified or optimized viral capsid variants. The research team embarked on an extensive screening process, utilizing AAV capsid libraries to isolate specific variants with enhanced binding and transduction efficiency for vascular endothelial cells. This innovative approach represents a significant leap forward in the ongoing quest for optimizing viral vectors for gene therapy.
In order to achieve their research objectives, the authors implemented various methodologies including in vivo evaluations in non-human primates. This approach is crucial as it provides a more accurate reflection of how these modified AAV variants might behave in human subjects. The use of non-human primates also helps to bridge the gap between preclinical studies and eventual clinical applications, ensuring that the findings are not only effective in laboratory settings but also viable in more complex biological systems.
The results of the study are promising, indicating a successful identification of novel AAV variants that demonstrated significantly improved transduction capacities in human vascular endothelial cells. This enhancement is crucial for the potential treatment of vascular diseases, where targeted gene therapy could revolutionize how such conditions are approached. The ability to efficiently deliver therapeutic genes directly into endothelial cells could lead to more effective interventions, reducing the risk of off-target effects and increasing the precision of therapeutic outcomes.
Furthermore, the study delves into the mechanisms that underlie the improved transduction observed with these novel AAV variants. By analyzing the interaction between the modified AAV capsids and the endothelial cell receptors, the researchers elucidated how certain mutations in the capsid proteins facilitate enhanced cellular uptake and gene expression. These insights can inform future research directives aimed at crafting even more potent AAV variants for diverse applications in gene therapy.
An important aspect of the research is its implications for the broader field of cardiovascular medicine. Cardiovascular diseases pose significant health burdens globally, and the ability to directly modify the endothelial cells responsible for vascular function presents a new frontier in treatment options. Gene therapy utilizing optimized AAVs could pave the way for innovative strategies to combat a range of vascular disorders, including atherosclerosis and hypertension, thereby potentially saving countless lives.
In addition to cardiovascular applications, the findings also open doors for other therapeutic realms, further emphasizing the versatility of AAV vectors. The study’s implications could resonate in various fields, including regenerative medicine, where targeted gene delivery may enhance tissue repair processes. The prospects of utilizing modified AAVs extend to a myriad of diseases, suggesting a bright future for gene therapy as a whole.
The authors also addressed the potential challenges and ethical considerations of using non-human primates for their research. This aspect of the study highlights the necessity of adhering to ethical guidelines while pushing the boundaries of scientific discovery. The careful selection of non-human primates as models serves to ensure that the research is conducted responsibly while still adhering to the rigor and validity required for such groundbreaking work.
In summary, Stamataki and colleagues have contributed substantially to the landscape of genetic research through their identification of AAV variants with enhanced transduction properties. By pioneering techniques that incorporate AAV capsid libraries and validate findings in non-human primates, they have set a precedent for future investigations into gene therapy. The implications of their work extend far beyond endothelial cells and could revolutionize therapeutic strategies across various medical sectors.
As the field of gene therapy continues to evolve, studies like this are essential for addressing the complex challenges posed by disease treatment and prevention. The pursuit of optimized AAV variants serves as a testament to the innovative spirit of researchers dedicated to harnessing the power of viral vectors for the betterment of human health. With ongoing advancements and discoveries, the horizon of gene therapy appears increasingly optimistic, holding the potential to transform clinical practice and significantly improve patient outcomes.
The research conducted signifies a monumental shift in the gene therapy paradigm, emphasizing the importance of engineering viral vectors that not only deliver genes effectively but also do so with precision and efficiency. As we look forward to the future of gene therapy, the contributions of Stamataki et al. will undoubtedly resonate within the scientific community and inspire a new generation of innovative research aimed at treating some of the most challenging diseases.
In conclusion, the identification of AAV variants with better transduction capabilities marks a critical development in the ongoing search for more effective gene therapy modalities. As the scientific community builds upon these findings, we are reminded of the endless possibilities inherent in the intersection of virology, genetics, and medicine, and how such synergy can ultimately lead to transformative therapies that have far-reaching impacts on humanity.
Subject of Research: Enhanced transduction of human vascular endothelial cells using identified AAV variants.
Article Title: Correction: Identification of AAV variants with improved transduction of human vascular endothelial cells by screening AAV capsid libraries in non-human primates.
Article References: Stamataki, M., Lüschow, J., Schlumbohm, C. et al. Correction: Identification of AAV variants with improved transduction of human vascular endothelial cells by screening AAV capsid libraries in non-human primates. Gene Ther (2025). https://doi.org/10.1038/s41434-025-00565-2
Image Credits: AI Generated
DOI: 10.1038/s41434-025-00565-2
Keywords: AAV variants, gene therapy, vascular endothelial cells, transduction efficiency, capsid libraries.