The small protein ubiquitin is involved in almost every cellular process in our body: it orchestrates the stability and function of the vast majority of proteins. When it binds to other proteins, they are often released for degradation. However, this labelling can also be reversed by special enzymes. The enzyme USP28, for example, is known to stabilise proteins that are important for cell growth and division – these can also play an important role in cancer growth.

Credit: Kisker/JMU
The small protein ubiquitin is involved in almost every cellular process in our body: it orchestrates the stability and function of the vast majority of proteins. When it binds to other proteins, they are often released for degradation. However, this labelling can also be reversed by special enzymes. The enzyme USP28, for example, is known to stabilise proteins that are important for cell growth and division – these can also play an important role in cancer growth.
In order to reduce the stability of these proteins and thus inhibit cancer growth, inhibitors of USP28 have been developed. These inhibitors, which form the basis of many anti-cancer drugs currently in development, disrupt cell division by blocking the USP28 enzyme. The problem is that they often act not only against USP28 but also against USP25, a closely related enzyme that separates ubiquitin from other proteins and is considered a key protein of the immune system. Further development of USP28 inhibitors into therapeutics that can be used in the clinic is therefore very difficult because of the foreseeable side effects – ranging from gastrointestinal problems to nerve damage and even autoimmune diseases.
Risk of Confusion Between the Two Enzymes
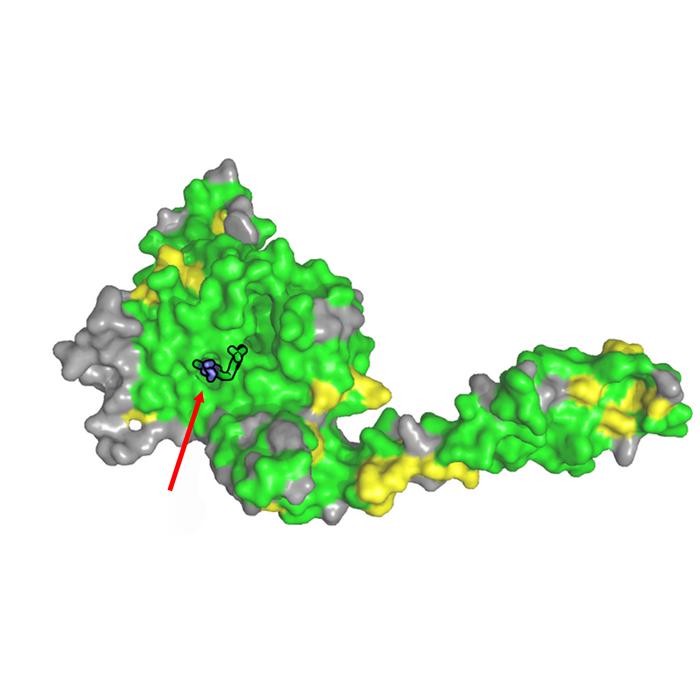
Researchers at the University of Würzburg (JMU) have now discovered why inhibitors not only target USP28, but also USP25: “Apparently, there is a high risk of confusion between USP28 and USP25“, explains Caroline Kisker, Chair of Structural Biology at the Rudolf Virchow Centre in Würzburg and Vice President for Research and Young Scientists. “We have been able to show that the two enzymes are very similar or even identical in many areas, including precisely where the inhibitors act.“
As part of her research, the biochemist’s team used X-ray crystallography to analyse the structure of USP28 in combination with the three inhibitors “AZ1“, “Vismodegib“ and “FT206“ – and thus determine the spatial binding site. Further biochemical experiments on USP25 showed that the sites where the inhibitors bind to USP28 and USP25 are identical. “The inhibitors are therefore unable to distinguish where they bind“, says Kisker. “This explains the non-specific effect.“
Discovery Paves the Way for the Development of Precise Inhibitors
The new scientific findings provide an important basis for the search for more specific drugs with fewer side effects. Their development is the next major goal of the Würzburg researchers. “Our structural biology data allows us to modify existing inhibitors so that they only work against either USP25 or USP28“, says Kisker. “We also want to look for inhibitors that bind to less similar enzyme sites. This will give these molecules greater targeting precision.“
The research was funded by the German Research Foundation (DFG).
The Rudolf-Virchow-Centre in Würzburg
The Rudolf Virchow Centre (RVZ) for Integrative and Translational Imaging is an interdisciplinary research centre that focuses on the visualisation of elementary life processes – from the sub-nano to the macro scale. As a central institution of the University of Würzburg, the centre is currently home to 13 translational research groups and around 100 researchers investigating the molecular causes of health and disease.
Journal
EMBO Reports
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Structural basis for the bi-specificity of USP25 and USP28 inhibitors
Article Publication Date
30-May-2024
COI Statement
The authors declare no competing interests.